Chapter Headings
Key Messages
- Over the past 20 years, the rates of acute myocardial infarction in people with diabetes has decreased substantially. However, the burden of disease remains high because of the increased prevalence of diabetes.
- Diabetes and hyperglycemia are independent predictors of increased short- and long-term mortality, recurrent myocardial infarction, and the development of heart failure in patients with acute myocardial infarction.
- People with an acute myocardial infarction and hyperglycemia (random blood glucose >11.0 mmol/L) may receive antihyperglycemic therapy to maintain blood glucose levels between 7.0 to 10.0 mmol/L.
- People with diabetes are less likely to receive recommended treatment, such as an early invasive strategy and revascularization, reperfusion therapy, beta blockers or dual antiplatelet therapy than people without diabetes. Efforts should be directed at promoting adherence to existing proven therapies in the high-risk person with myocardial infarction and diabetes.
Key Messages for People with Diabetes
- A heart attack can manifest as chest discomfort or crushing pain; or as pain in the arms, back, neck, jaw and, even, the stomach. Shortness of breath, cold sweat, nausea and lightheadedness may also occur.
- If you are experiencing symptoms of a heart attack, you should seek medical help immediately. The faster treatment is started, the better.
Introduction
Diabetes (together with lipid abnormalities, smoking and hypertension) is one of the top 4 independent risk factors for myocardial infarction (MI) (1). Today, approximately 15% to 35% of people admitted with an acute coronary syndrome (ACS) have known diabetes (2), and as many as a further 15% have undiagnosed diabetes (3). Between 1990 and 2010, there was a 67.8% reduction of the rates of acute MI in people with diabetes, compared to a 32% reduction in individuals without diabetes (4). However, as a result of the substantial increase in the prevalence of diabetes over this period, the public health burden of MI in people with diabetes continues to rise.
Compared to individuals without diabetes, people with diabetes have:
- A 3-fold increased risk of ACS (5)
- Occurrence of acute coronary events 15 years earlier (5)
- A 2-fold increased short- (6,7) and long-term mortality (6,8)
- An increased incidence of post-infarction recurrent ischemic events, heart failure and cardiogenic shock (3,9)
- A similar benefit from guideline-recommended management strategies (see below)
- Less utilization of guideline recommended care (10–13), including an invasive strategy (14) which may contribute to adverse outcomes (15).
Risk Stratification of People With Diabetes and ACS
It is recognized that there is a wide range of risk for an adverse outcome in people with diabetes after an ACS. A recent study developed a prediction model that indicated age, renal dysfunction, the presence of anemia, heart failure or left ventricular (LV) dysfunction, in-hospital revascularization, obesity, prior ACS and insulin treatment were factors significantly associated with mortality during the 5 years after acute MI (AMI) (16).
Identification of Diabetes in People with ACS
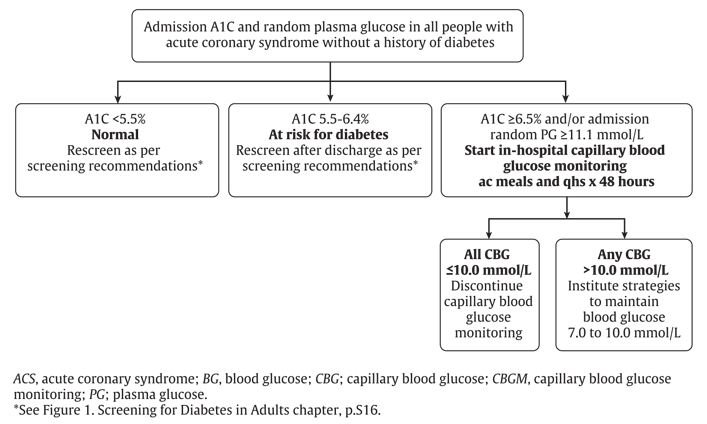
Although the absolute number of people with MI has fallen in the United States, the prevalence of diabetes in this population has steadily increased from 18% in 1997 to 30% in 2006 (16). More than two-thirds of people with MI have either diabetes or prediabetes (impaired glucose tolerance [IGT] or impaired fasting glucose [IFG]) (17). Abnormal glucose regulation is almost twice as prevalent in people with MI compared to a matched control population and is a marker for adverse outcomes (18). The frequency of previously unrecognized diabetes in the ACS population is reported to be between 4% and 22% depending on the test used for the diagnosis of diabetes (3,19). If fasting plasma glucose (FPG) criteria is used alone in the ACS population, diabetes is underdiagnosed in 39% compared to when the diagnosis is made from an oral glucose tolerance test (OGTT) (20). An A1C >6.5% is currently a diagnostic criterion for diabetes as it captures long-term glucose exposure, does not require fasting or timed samples and is currently used to guide management decisions (see Screening for Diabetes in Adults chapter, p. S16). One study has validated the use of A1C in an acute care population and found that using the 2-hour 75 g OGTT as a gold standard for the diagnosis of diabetes, and an A1C threshold of 6.0%, A1C had a sensitivity of 77% and a specificity of 87% (21). It is accepted that some people with diabetes will be missed by screening with fasting plasma glucose (FPG) and A1C compared to the universal use of an OGTT. However, it is likely that the people most in need of glycemic control will be detected with these simple tests that can be widely applied. In-hospital capillary blood glucose monitoring should be started in individuals without a history of diabetes with an admission A1C ≥6.5% or random plasma glucose (PG) >10.0 mmol/L. Individuals with an A1C between 5.5% to 6.4% should have repeat screening after discharge as per diabetes screening guidelines (see Screening for Diabetes in Adults chapter, p. S16 and Figure 1
Management of ACS in People With Diabetes
Guidelines for the management of people with ACS have been developed by the American College of Cardiology/American Heart Association (22–24) and the European Society of Cardiology (25,26). In most situations, there are no clinical trials that specifically address management of people with diabetes and ACS; however, subgroup analyses in people with diabetes and ACS show either a similar or enhanced benefit from treatment compared to the overall group for: a) reperfusion with fibrinolysis (27) or primary angioplasty (28) for ST-segment elevation ACS; and b) an early invasive strategy (29) with the use of dual anti-platelet therapy with acetylsalicylic acid (ASA) and clopidogrel (30), glycoprotein IIb/IIIa inhibitors and the newer P2Y12 platelet inhibitors (prasugrel and ticagrelor) in people with non-ST segment elevation ACS at high risk of recurrent ischemic events (31).
A significant care gap exists for people with diabetes not receiving guideline-recommended treatment compared to people without diabetes (10–12,15,16). It is possible that the underutilization of recommended treatment is one factor contributing to the adverse outcome of the person with diabetes and ACS.
Anti-Platelet Therapy and ACS in People With Diabetes
Platelet aggregation plays a central role in the development of the occlusive thrombus responsible for acute coronary occlusion in people with ACS. People with diabetes have a pro-thrombotic state due to dysfunctional and hyperactive platelets, endothelial dysfunction, elevated coagulation factors and decreased fibrinolysis (32). Increased platelet activity is due to multiple metabolic and cellular factors associated with diabetes that include endothelial dysfunction, the impact of hyperglycemia and deficient insulin action (32).
Diabetes is associated with an increased incidence of recurrent atherothrombotic events (33), including stent thrombosis (34). Anti-platelet therapy has been shown to reduce atherothrombotic events in people with ACS, both during the acute phase and in the longer term. The beneficial effect of ASA has been shown in multiple clinical trials in patients with non–ST-segment elevation acute coronary syndrome (NSTE ACS) and ST-segment elevation MI (STEMI). The Antithrombotic Trialist's Collaboration meta-analysis (35) of anti-platelet therapy (mainly ASA) included 212,000 high-risk participants (with acute or previous vascular disease) and showed the incidence of vascular events to be reduced in both the overall population (16.8% to 12.8%; p<0.00001) and in the participants with diabetes (22.3% to 18.5%; p<0.002). Low-dose ASA (75 to 150 mg) was as effective as higher doses (>150 mg) with a lower incidence of bleeding complications. The Clopidogrel optimal loading dose Usage to Reduce Recurrent EveNTs-Organization to Assess Strategies in Ischemic Syndromes (CURRENT/OASIS 7) trial (36) also was unable to show any benefit from higher dose compared to low-dose (75 to 100 mg) ASA in people with and without diabetes. The use of low-dose ASA is recommended to minimize GI bleeding in people with and without diabetes (see Cardiovascular Protection in People with Diabetes chapter, p. S162).
Dual anti-platelet therapy with ASA and clopidogrel, administered from the time of presentation, has been the recommended standard of care for people with NSTE ACS. People with diabetes in the Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial (30) had a similar benefit with clopidogrel vs. placebo (14.2% vs. 17.7%, RR 0.84, 95% CI 0.70–1.02) as the overall population (9.3% vs. 11.4%, RR 0.80, 95% CI 0.72–0.90). Despite dual-antiplatelet therapy with ASA and clopidogrel, recurrent atherothrombotic events continue to occur, especially in the person with diabetes. Clopidogrel is a relatively weak inhibitor of platelet aggregation with a wide variation of inhibition of in-vitro platelet aggregation. There is a higher incidence of events in people with residual platelet activity and people with diabetes have higher residual platelet activity despite ASA and clopidogrel treatment. Two more potent antiplatelet agents, prasugrel and ticagrelor, that are more effective and predictable inhibitors of platelet aggregation, have been shown to improve outcomes, especially in people with diabetes.
In the TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel - Thrombolysis In Myocardial Infarction (TRITON-TIMI 38) trial, prasugrel administered at the time of coronary angioplasty in participants with ACS reduced recurrent ischemic events, including stent thrombosis, compared to participants receiving clopidogrel (37). In subjects with diabetes, prasugrel treatment was associated with greater platelet inhibition and fewer poor responders (38). Prasugrel resulted in an important net clinical benefit in people with diabetes (39) (14.6 vs. 19.2%, HR 0.74, p=0.001) due to a 30% reduction of the primary endpoint (cardiovascular [CV]) death, non-fatal MI or stroke over the 14.4 months of the study. In this subgroup with diabetes, there was no significant increase in major bleeding. There was no statistical interaction between the subgroups with and without diabetes, indicating that the enhanced absolute benefit was the result of higher event rates in people with diabetes.
In the Platelet Inhibition and Patient Outcomes (PLATO) trial, the P2Y
The availability of more potent and reliable anti-platelet agents for the management of people with ACS provides an opportunity to further reduce recurrent ACS and mortality. High-risk people with diabetes with either STEMI or NSTE ACS should be considered for treatment with either prasugrel (after the coronary disease anatomy has been defined) or ticagrelor.
Platelet aggregation is largely mediated by the glycoprotein (GP) IIb/IIIa receptor through its binding to fibrinogen. The GPIIb/IIIa receptor inhibitors abciximab, eptifibatide and tirofiban were shown to be effective for the management of ACS in people with diabetes in a meta-analysis of 6 clinical trials. GPIIb/IIIa inhibitors were shown to reduce 30-day mortality by 26% (4.6% vs. 2.6%, p=0.007) (31). In contrast, people without diabetes had no mortality benefit. Although these trials were performed in an era before dual anti-platelet therapy with ASA and clopidogrel was used, studies (42,43) indicate an additional benefit from a GPIIb/IIIa inhibitor for people with high-risk ACS, such as those with diabetes who are undergoing percutaneous coronary intervention (PCI). However, these benefits have not been observed when more potent oral anti-platelet agents, such as ticagrelor, are used (44).
More prolonged duration dual anti-platelet therapy with ASA and ticagrelor in people with ACS, administered for up to 3 years beyond the usual 1-year treatment, was shown in the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial to reduce the primary endpoint of non-fatal MI, stroke or CV death (placebo 9.04% ticagrelor 60 mg 7.77% (hazard ratio [HR] 0.84, 95% CI 0.74–0.95), ticagrelor 90 mg 7.85% (HR 0.85, 95% CI 0.75–0.96) (44). There was no advantage to receiving ticagrelor 90 mg twice daily rather than 60 mg twice daily, and major bleeding was slightly more at the higher dose (placebo 1.06%, ticagrelor 60 mg 2.3%, ticagrelor 90 mg 2.6%). Participants with diabetes receiving ticagrelor, had a similar relative risk reduction of the primary combined endpoint as the overall group (45). However, with a 50% higher event rate, those with diabetes had an 60% greater absolute benefit than the participants without diabetes (participants with diabetes: placebo 11.6%, ticagrelor 60 mg twice daily 10.0% [HR 0.83, 95% CI 0.69–1.00]; participants without diabetes: placebo 7.8%, ticagrelor 6.7% [HR 0.84, 95% CI 0.72–0.98]). The increased bleeding rates with ticagrelor were similar in the people with diabetes to those without diabetes. People at very high risk or recurrent ischemic events (such as people with extensive coronary artery disease [CAD] not completely revascularized, or recurrent ACS despite usual recommended treatment) and with a low or average bleeding risk, should be considered for prolonged (up to 3 years post-ACS) treatment with ticagrelor 60 mg twice daily.
Glycemic Control
Hyperglycemia during the first 24 to 48 hours after admission for ACS is associated with an increased early mortality, whether or not the person has diabetes (46,47). Furthermore, in-hospital mortality has a closer relationship to hyperglycemia than to diabetic status (48,49). Higher baseline blood glucose (BG) and a failure of BG to decrease are independent predictors of mortality (50). For people undergoing primary angioplasty, mortality increases when the plasma glucose (PG) is >10.0 mmol/L (47).
Although elevated mean BG level in the first 24 hours after onset of ACS is associated with adverse outcomes (51), evidence to support reducing BG levels (especially to levels close to the normal range) after ACS, remains inconclusive. The Diabetes Mellitus Insulin Glucose Infusion in Acute Myocardial Infarction (DIGAMI 1) study indicated that tight glycemic control with the use of intravenous insulin in the early hours after presentation, followed by multidose subcutaneous insulin treatment over the subsequent months, resulted in a 30% reduction in 1-year mortality (52–56). The DIGAMI 2 study failed to achieve the study goals, both in the number of participants recruited and in glycemic targets (52). However, despite these limitations, it did demonstrate that outcomes were closely related to glycemic control, however achieved. Studies have shown that glucose-insulin-potassium infusion in patients with AMI do not improve outcomes; however, these protocols often resulted in increased BG levels and, therefore, cannot be used as evidence for outcomes associated with glycemic control. In the Hyperglycemia: Intensive Insulin Infusion in Infarction (HI-5) study of glucose and insulin in people with AMI, participants with a blood glucose maintained at <8.0 mmol/L had lower mortality than subjects with higher levels (57).
In conclusion, clinical trial data do not conclusively show that tight glycemic control early after an ACS improves long-term outcomes. Furthermore, the impact of hypoglycemia may negate any potential benefit. Glycemic control in the post MI patient should be consistent with the Diabetes Canada clinical practice guidelines recommendations for management of hyperglycemia in the hospitalized patient (see In-Hospital Management of Diabetes chapter, p. S115).
Revascularization
ACS practice guidelines promote the same treatment strategies in people with diabetes as for those without diabetes (58) . An early invasive strategy with revascularization when possible in non-ST elevation (NSTE) ACS provides a similar or greater reduction in death and MI (up to 5 years of follow up) in the subset of participants with diabetes compared to the overall population (27,59,60). An early invasive, rather than a selective invasive (conservative), strategy is recommended, in the absence of contraindications in people with diabetes and a NSTE ACS.
Trials comparing coronary artery bypass grafting (CABG) and PCI in people with diabetes with stable multivessel disease or ACS have provided consistent results in favour of CABG (61) with improved outcomes of death, MI and repeat revascularization, despite an excess of stroke in people undergoing CABG. These results are generally extrapolated to the higher-risk ACS population with diabetes with NSTE-ACS and complex coronary anatomies. Therefore, CABG with the use of internal thoracic artery bypass should be the preferred revascularization modality over complex PCI in light of the consistent results in randomized trials with the provision that patient characteristics (such as frailty, cerebrovascular disease, among others) need to be considered. Percutaneous coronary interventions (with newer generation drug-eluting stents whenever possible) is acceptable for people with less extensive disease (i.e. single-vessel disease or 2-vessel disease without involvement of the left anterior descending (LAD) and those with Synergy Between PCI With Taxus and Cardiac Surgery (SYNTAX) score ≤22) (62).
For people with ST-elevation ACS, immediate reperfusion strategies with either fibrinolysis or primary PCI (PPCI) result in similar benefits for people with and without diabetes. The benefits of PPCI over fibrinolysis in people with diabetes are similar to those in the population without diabetes (Odds ratio [OR] mortality with primary PCI vs. fibrinolysis in people with diabetes 0.49 [95% CI 0.31–0.79]) (27). However, fibrinolysis should be administered when PPCI is not available, within acceptable timeframes. Ocular hemorrhage in people with diabetic retinopathy is extremely rare and should not limit the use of fibrinolysis when it is indicated (59).
Recommendations
- In all people with ACS, a random BG and an A1C (if not done in the 3 months prior to admission) should be measured:
- For people with a history of diabetes, to identify individuals that would benefit from glycemic optimization [Grade D, Consensus]
- For people without a history of diabetes, to identify individuals at risk for ongoing dysglycemia [Grade D, Consensus]
- If the A1C is ≥6.5% and/or random BG is >11.0 mmol/L, in-hospital capillary blood glucose monitoring should be initiated [Grade D, Consensus]
- If A1C is 5.5–6.4%, repeat screening for diabetes should be performed after discharge as per diabetes screening recommendations [Grade D, Consensus]) (see Figure 1. Screening for Diabetes in Adults chapter, p. S16).
- In-hospital management of diabetes in ACS should include strategies to avoid both hyperglycemia and hypoglycemia:
- People with ACS and a random BG of >11.0 mmol/L on admission may be treated to achieve BG levels in the range of 7.0–10.0 mmol/L followed by strategies to achieve recommended BG targets long term [Grade C, Level 2 (52,55)]. Insulin therapy may be required to achieve these targets [Grade D, Consensus]
- An appropriate protocol should be developed and staff trained to ensure the safe and effective implementation of this therapy and to minimize the likelihood of hypoglycemia [Grade D, Consensus].
- People with diabetes and ACS should receive the same treatments that are recommended for people with ACS without diabetes since they benefit equally [Grade D, Consensus].
- In people with diabetes and ACS undergoing PCI, antiplatelet therapy with prasugrel (if clopidogrel naïve, <75 years of age, weight >60 kg, and no history of stroke) [Grade A, Level 1 (37,39)] or ticagrelor [Grade B, Level 1 (40,41)], rather than clopidogrel, should be used to further reduce recurrent ischemic events. People with diabetes and non-STE ACS and higher risk features destined for a selective invasive strategy should receive ticagrelor, rather than clopidogrel [Grade B, Level 2 (40,41)]
- In people with diabetes and ACS, at very high risk of recurrent ischemic events and at average or low bleeding risk, prolonged (up to 3 years post ACS) treatment with ticagrelor 60 mg twice daily should be considered [Grade B, Level 2 (45)]
- In people with diabetes and non-STE ACS and high risk features, an early invasive approach, rather than a selective invasive approach to revascularization, should be used to reduce recurrent coronary events, unless contraindicated [Grade B, Level 2 (29)]
- For people with diabetes with NSTE-ACS and complex coronary anatomy, CABG should be considered rather than complex PCI [Grade A, Level 1 (62)]
- In people with diabetes and STE-ACS, the selection of the reperfusion modality (PPCI vs. fibrinolysis) should not differ from people with STE-ACS without diabetes; the presence of retinopathy should not be a contraindication to fibrinolysis [Grade B, Level 2 (59)].
Abbreviations:
A1C, glycated hemoglobin; ACS, acute coronary syndrome; AMI, acute myocardial infarction; ASA, acetylsalicylic acid; BG, blood glucose; CABG, coronary artery bypass grafting; CI, confidence interval; CV, cardiovascular; FPG, fasting plasma glucose; HR, hazard ratio; IGT, impaired glucose tolerance; LV, left ventricular; MI, myocardial infarction; NSTE, non-ST-elevation; OGTT, oral glucose tolerance test; OR, odds ratio; PCI, percutaneous coronary intervention; PG, plasma glucose; PPCI, primary percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Other Relevant Guidelines
-
In-Hospital Management of Diabetes, p. S115.
Literature Review Flow Diagram for Chapter 27: Management of Acute Coronary Syndromes
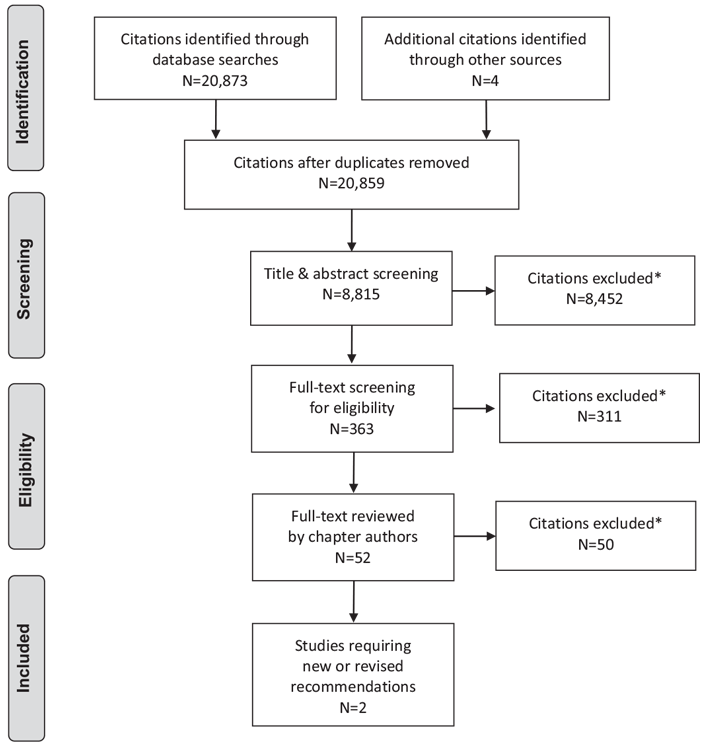
*Excluded based on: population, intervention/exposure, comparator/control or study design.
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi:10.1371/journal.pmed1000097 (63).
For more information, visit www.prisma-statement.org.
Author Disclosures
Dr. L.-L'Allier reports minor personal fees from Philips (Volcano) and Abbott-SJM, while contributing to the guidelines. Dr. Fitchett reports personal fees from AstraZeneca, Sanofi, and Lilly.
References
- Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004;364:937–52.
- Ovbiagele B, Markovic D, FonarowGC. Recent US patterns and predictors of prevalent diabetes among acute myocardial infarction patients. Cardiol Res Pract 2011;2011:145615.
- Aguilar D, Solomon SD, Kober L, et al. Newly diagnosed and previously known diabetes mellitus and 1-year outcomes of acute myocardial infarction: The VALsartan In Acute myocardial iNfarcTion (VALIANT) trial. Circulation 2004;110:1572–8.
- Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med 2014;370:1514–23.
- Booth GL, Kapral MK, Fung K, et al. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: A population-based retrospective cohort study. Lancet 2006;368:29–36.
- Donahoe SM, Stewart GC, McCabe CH, et al. Diabetes and mortality following acute coronary syndromes. JAMA 2007;298:765–75.
- Behar S, Boyko V, Reicher-Reiss H, et al. Ten-year survival after acute myocardial infarction: Comparison of patients with and without diabetes. SPRINT study group. Secondary Prevention Reinfarction Israeli Nifedipine Trial. Am Heart J 1997;133:290–6.
- Kumler T, Gislason GH, Kober L, et al. Diabetes is an independent predictor of survival 17 years after myocardial infarction: Follow-up of the TRACE registry. Cardiovasc Diabetol 2010;9:22.
- Kannel WB, Thomas HE Jr. Sudden coronary death: The framingham study. Ann N Y Acad Sci 1982;382:3–21.
- Hasin T, Hochadel M, Gitt AK, et al. Comparison of treatment and outcome of acute coronary syndrome in patients with versus patients without diabetes mellitus. Am J Cardiol 2009;103:772–8.
- Hung J, Brieger DB, Amerena JV, et al. Treatment disparities and effect on late mortality in patients with diabetes presenting with acute myocardial infarction: Observations from the ACACIA registry. Med J Aust 2009;191:539–43.
- Norhammar A, Lindback J, Ryden L, et al. Improved but still high short- and longterm mortality rates after myocardial infarction in patients with diabetes mellitus: A time-trend report from the Swedish register of information and knowledge about swedish heart intensive care admission. Heart 2007;93:1577– 83.
- Brogan GX Jr, Peterson ED, Mulgund J, et al. Treatment disparities in the care of patients with and without diabetes presenting with non-ST-segment elevation acute coronary syndromes. Diabetes Care 2006;29:9–14.
- Gustafsson I, Hvelplund A, Hansen KW, et al. Underuse of an invasive strategy for patients with diabetes with acute coronary syndrome: A nationwide study. Open Heart 2015;2:e000165.
- Yan RT, Yan AT, TanM, et al. Underuse of evidence-based treatment partly explains the worse clinical outcome in diabetic patients with acute coronary syndromes. Am Heart J 2006;152:676–83.
- Arnold SV, Spertus JA, Jones PG, et al. Predicting adverse outcomes after myocardial infarction among patients with diabetes mellitus. Circ Cardiovasc Qual Outcomes 2016;9:372–9.
- Bartnik M, Ryden L, Ferrari R, et al. The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe. The Euro heart survey on diabetes and the heart. Eur Heart J 2004;25:1880–90.
- Bartnik M, Malmberg K, Hamsten A, et al. Abnormal glucose tolerance–a common risk factor in patients with acute myocardial infarction in comparison with population-based controls. J Intern Med 2004;256:288–97.
- Mozaffarian D, Marfisi R, Levantesi G, et al. Incidence of new-onset diabetes and impaired fasting glucose in patients with recent myocardial infarction and the effect of clinical and lifestyle risk factors. Lancet 2007;370:667–75.
- Bartnik M, Ryden L, Malmberg K, et al. Oral glucose tolerance test is needed for appropriate classification of glucose regulation in patients with coronary artery disease: A report from the Euro heart survey on diabetes and the heart. Heart 2007;93:72–7.
- Silverman RA, Thakker U, Ellman T, et al. Hemoglobin A1c as a screen for previously undiagnosed prediabetes and diabetes in an acute-care setting. Diabetes Care 2011;34:1908–12.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction- executive summary: A report of the American college of cardiology/ American heart association task force on practice guidelines (writing committee to revise the 2002 guidelines for the management of patients with unstable Angina/Non-ST-elevationmyocardial infarction): Developed in collaboration with the American college of emergency physicians, American college of physicians, society for academic emergency medicine, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J Am Coll Cardiol 2007;50:652–726.
- Kushner FG, Hand M, Smith SC Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American college of cardiology foundation/ American heart association task force on practice guidelines. J Am Coll Cardiol 2009;54:2205–41.
- Wright RS, Anderson JL, Adams CD, et al. 2011 ACCF/AHA focused update of the guidelines for the management of patients with unstable angina/non-STelevation myocardial Infarction (updating the 2007 guideline): A report of the American college of cardiology foundation/American heart association task force on practice guidelines developed in collaboration with the American college of emergency physicians, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J Am Coll Cardiol 2011;57:1920–59.
- Van de Werf F, Bax J, Betriu A, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: The task force on the management of ST-segment elevation acute myocardial infarction of the European society of cardiology. Eur Heart J 2008;29:2909–45.
- Task Force for Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of European Society of Cardiology, Bassand JP, Hamm CW, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007;28:1598–660.
- Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: Collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet 1994;343:311–22.
- Timmer JR, van der Horst IC, de Luca G, et al. Comparison of myocardial perfusion after successful primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction with versus without diabetes mellitus. Am J Cardiol 2005;95:1375–7.
- O’Donoghue ML, Vaidya A, Afsal R, et al. An invasive or conservative strategy in patients with diabetes mellitus and non-ST-segment elevation acute coronary syndromes: A collaborative meta-analysis of randomized trials. J Am Coll Cardiol 2012;60:106–11.
- Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494–502.
- Roffi M, Chew DP, Mukherjee D, et al. Platelet glycoprotein IIb/IIIa inhibitors reduce mortality in diabetic patients with non-ST-segment-elevation acute coronary syndromes. Circulation 2001;104:2767–71.
- Ferreiro JL, Angiolillo DJ. Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation 2011;123:798–813.
- Malmberg K, Yusuf S, Gerstein HC, et al. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: Results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) registry. Circulation 2000;102:1014–19.
- Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005;293:2126–30.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71–86.
- Mehta SR, Tanguay JF, Eikelboom JW, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENTOASIS 7): A randomised factorial trial. Lancet 2010;376:1233–43.
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–15.
- Angiolillo DJ, Badimon JJ, Saucedo JF, et al. A pharmacodynamic comparison of prasugrel vs. high-dose clopidogrel in patients with type 2 diabetes mellitus and coronary artery disease: Results of the Optimizing anti-Platelet Therapy In diabetes MellitUS (OPTIMUS)-3 Trial. Eur Heart J 2011;32:838–46.
- Wiviott SD, Braunwald E, Angiolillo DJ, et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-Thrombolysis in myocardial infarction 38. Circulation 2008;118:1626–36.
- Cannon CP, Harrington RA, James S, et al. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): A randomised double-blind study. Lancet 2010;375:283–93.
- James S, Angiolillo DJ, Cornel JH, et al. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: A substudy from the PLATelet inhibition and patient outcomes (PLATO) trial. Eur Heart J 2010;31:3006–16.
- Kastrati A, Mehilli J, Neumann FJ, et al. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: The ISAR-REACT 2 randomized trial. JAMA 2006;295:1531–8.
- De Luca G, Navarese E, Marino P. Risk profile and benefits fromGp IIb-IIIa inhibitors among patients with ST-segment elevation myocardial infarction treated with primary angioplasty: A meta-regression analysis of randomized trials. Eur Heart J 2009;30:2705–13.
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–57.
- Bhatt DL, Bonaca MP, Bansilal S, et al. Reduction in ischemic events with ticagrelor in diabetic patients with prior myocardial infarction in PEGASUS-TIMI 54. J Am Coll Cardiol 2016;67:2732–40.
- Angeli F, Verdecchia P, Karthikeyan G, et al. New-onset hyperglycemia and acute coronary syndrome: A systematic overview and meta-analysis. Curr Diabetes Rev 2010;6:102–10.
- Kosiborod M, Rathore SS, Inzucchi SE, et al. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: Implications for patients with and without recognized diabetes. Circulation 2005;111:3078– 86.
- Goyal A, Mehta SR, Gerstein HC, et al. Glucose levels compared with diabetes history in the risk assessment of patients with acute myocardial infarction. Am Heart J 2009;157:763–70.
- Kosiborod M, Inzucchi SE, Krumholz HM, et al. Glucometrics in patients hospitalized with acute myocardial infarction: Defining the optimal outcomesbased measure of risk. Circulation 2008;117:1018–27.
- Goyal A, Mahaffey KW, Garg J, et al. Prognostic significance of the change in glucose level in the first 24 h after acute myocardial infarction: Results from the CARDINAL study. Eur Heart J 2006;27:1289–97.
- Porter A, Assali AR, Zahalka A, et al. Impaired fasting glucose and outcomes of ST-elevation acute coronary syndrome treated with primary percutaneous intervention among patients without previously known diabetes mellitus. Am Heart J 2008;155:284–9.
- Malmberg K, Ryden L, Wedel H, et al. Intense metabolic control by means of insulin in patients with dSiabetes mellitus and acute myocardial infarction (DIGAMI 2): Effects on mortality and morbidity. Eur Heart J 2005;26:650–61.
- Malmberg K, Ryden L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): Effects on mortality at 1 year. J Am Coll Cardiol 1995;26:57–65.
- Malmberg K, Rydén L, Hamsten A, et al. Effects of insulin treatment on causespecific one-year mortality and morbidity in diabetic patients with acute myocardial infarction. DIGAMI study group. Diabetes Insulin-Glucose in Acute Myocardial Infarction. Eur Heart J 1996;17:1337–44.
- Malmberg K. Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) study group. BMJ 1997;314:1512–15.
- Malmberg KA, Efendic S, Ryden LE. Feasibility of insulin-glucose infusion in diabetic patients with acute myocardial infarction. A report from the multicenter trial: DIGAMI. Diabetes Care 1994;17:1007–14.
- Cheung NW,Wong VW, McLean M. The hyperglycemia: Intensive Insulin Infusion in Infarction (HI-5) study: A randomized controlled trial of insulin infusion therapy for myocardial infarction. Diabetes Care 2006;29:765–70.
- Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC), European Association for Cardio-Thoracic Surgery (EACTS), European Association for Percutaneous Cardiovascular Interventions (EAPCI), Wijns W, Kolh P, Danchin N, et al. Guidelines onmyocardial revascularization. Eur Heart J 2010;31:2501–55.
- Mahaffey KW, Granger CB, Toth CA, et al. Diabetic retinopathy should not be a contraindication to thrombolytic therapy for acutemyocardial infarction: Review of ocular hemorrhage incidence and location in the GUSTO-I trial. Global Utilization of Streptokinase and t-PA for Occluded coronary arteries. J AmColl Cardiol 1997;30:1606–10.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012;367:2375– 84.
- Fox KA, Clayton TC, Damman P, et al. Long-term outcome of a routine versus selective invasive strategy in patients with non-ST-segment elevation acute coronary syndrome a meta-analysis of individual patient data. J Am Coll Cardiol 2010;55:2435–45.
- Verma S, Farkouh ME, Yanagawa B, et al. Comparison of coronary artery bypass surgery and percutaneous coronary intervention in patients with diabetes: A meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol 2013;1:317–28.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 2009;6:e1000097.
*The Canadian Diabetes Association is the registered owner of the name Diabetes Canada. All content on guidelines.diabetes.ca, CPG Apps and in our online store remains exactly the same. For questions, contact communications@diabetes.ca.