Chapter Headings
- Introduction
- Reducing the Risk of Developing Type 1 Diabetes
- Reducing the Risk of Developing Type 2 Diabetes
- Healthy Behaviour Interventions
- Medical Nutrition Therapy
- Dietary Patterns
- Physical Activity
- Pharmacotherapy
- Bariatric Surgery
- Diabetes Prevention in High-Risk Ethnicities
- Population Level Interventions for Prevention of Type 2 Diabetes
- Author Disclosures
Key Messages
- As safe and effective preventive therapies for type 1 diabetes have not yet been identified, any attempts to prevent type 1 diabetes should be undertaken only within the confines of formal research protocols.
- Intensive and structured healthy behaviour interventions, ideally resulting in loss of approximately 5% of initial body weight, can reduce the risk of progression from impaired fasting glucose or impaired glucose tolerance to type 2 diabetes by almost 60%. When initiated early, the effects of healthy behaviour interventions are long lasting (more than 20 years).
- Progression from prediabetes to type 2 diabetes can also be reduced by pharmacologic therapy with metformin (~30% reduction), with persistent benefits observed after more than 10 years of stopping treatment in the Diabetes Prevention Program.
Key Messages for People with Prediabetes
- If you have prediabetes, healthy behaviour changes that result in a loss of 5% of your initial body weight can delay or prevent type 2 diabetes from developing.
- A registered dietitian can educate you about dietary changes that may help reduce your risk for developing diabetes.
- Regular physical activity is also important to reduce your risk of diabetes.
- If healthy behaviour changes are not enough to normalize your blood glucose, your health-care provider may recommend that you use medication in addition to ongoing healthy behaviour changes to manage your prediabetes.
Introduction
Ideal prevention strategies for both type 1 and type 2 diabetes should range from efforts focused on individuals identified as being at risk for developing diabetes to broader group- and population-based strategies. Prevention or delay in the onset of diabetes should not only alleviate the burden of the disease on the individual, but could also decrease the associated morbidity and mortality. Ideal prevention strategies would differ depending on the type of diabetes. Given its increasing incidence and prevalence, the development of safe and cost-effective interventions to reduce the risk of developing diabetes are urgently needed to decrease the burden on individuals and the health-care system.
Reducing the Risk of Developing Type 1 Diabetes
Type 1 diabetes is a chronic autoimmune condition characterized by destruction of pancreatic beta cells. The causes are multi-factorial, with both genetic and environmental factors. The exact nature of causative environmental factors continues to be debated. There is a long preclinical period before the onset of overt symptoms, which may be amenable to therapeutic intervention to prevent disease. Immunotherapeutic interventions continue to be the main focus of type 1 diabetes prevention.
Two major trials of interventions to prevent or delay the onset of type 1 diabetes have been completed. The European Nicotinamide Diabetes Intervention Trial (ENDIT), a randomized, double-blind, placebo-controlled trial of high-dose nicotinamide therapy, recruited first-degree relatives of people who were <20 years of age when diagnosed with type 1 diabetes, islet cell antibody positive, <40 years of age and who had a normal oral glucose tolerance test (OGTT). Although nicotinamide was protective in animal studies, no effect was observed in ENDIT during the 5-year trial period (1). The Diabetes Prevention Trial-Type 1 (DPT-1) studied the efficacy of low-dose insulin injections in high-risk (projected 5-year risk of >50%) first-degree relatives of people with type 1 diabetes. Overall, the insulin treatments had no effect (2), but in a subset of participants with high levels of insulin autoantibodies, a delay, and perhaps a reduction, in the incidence of type 1 diabetes was observed (3). A third ongoing large trial, the Trial to Reduce IDDM in the Genetically at Risk (TRIGR) study, is investigating the effect of excluding cow's milk protein and replacing it with hydrolyzed formula milk in genetically at-risk infants until 6 to 8 months of age. Preliminary data showed no reduction in the development of diabetes antibodies at age 6 (4), but data on the overt development of diabetes by age 10 is not yet available (5).
A second strategy is to try to halt, at the time of diagnosis, the immune-mediated destruction of beta cells to preserve any residual capacity to produce insulin. Progress in the field has been slow due to safety considerations; namely, side effects from immunosuppression/modulation must be minimized before consideration can be given for clinical use, especially because of the reasonable life expectancy of people with type 1 diabetes and technological advancements with insulin replacement therapy.
As safe and effective preventive therapies for type 1 diabetes have not yet been identified, any attempts to prevent type 1 diabetes should be undertaken only within the confines of formal research protocols.
Reducing the Risk of Developing Type 2 Diabetes
Preventing type 2 diabetes may result in significant public health benefits, including lower rates of cardiovascular disease (CVD), renal failure, blindness and premature mortality (6). An epidemiological analysis projected that if all diabetes could be avoided in Caucasian American males through effective primary prevention, the risk of all-cause and cardiovascular (CV) mortality in the entire population could be reduced by up to 6.2% and 9.0%, respectively (7). Data from the United States indicates that 28% of CV expenditures are attributable to diabetes (8).
Primary approaches to preventing diabetes in a population include the following: 1) programs targeting high-risk individuals [such as those with impaired glucose tolerance (lGT), impaired fasting glucose (IFG), or obesity]; 2) programs targeting high-risk subgroups, such as high-risk ethnic groups; and 3) programs for the general population, such as those designed to promote physical activity and healthy eating in adults or children (9–11).
Prospective cohort studies have identified historical, physical and biochemical variables associated with the development of type 2 diabetes. These include older age, family history of type 2 diabetes, certain ethnic backgrounds, prediabetes, history of gestational diabetes, CVD and obesity (especially abdominal obesity), (12–14) and are detailed in Table 1 of the Screening for Diabetes in Adults chapter, p. S16. Results of large, well-designed studies assessing healthy behavior and pharmacologic interventions in adults to prevent the progression from IGT to diabetes have been published. No pharmacologic agent is currently approved for diabetes prevention in Canada. Recently, more data has emerged on the role of bariatric surgery in prevention of type 2 diabetes in high-risk groups; however, the cost-benefit analysis of surgical intervention remains questionable (15).
Healthy Behaviour Interventions
A majority of the randomized controlled trials with healthy behaviour interventions enrolled participants with IGT based on OGTT results. However, as the use of OGTT is diminishing clinically for screening for prediabetes and diabetes, and alternative methods including glycated hemoglobin (A1C) and fasting plasma glucose (FPG) are being used more frequently, the recommendations based on the following randomized controlled trials will be applied to a prediabetes diagnosis, irrespective of the testing method.
Healthy behaviour interventions were assessed in the Finnish Diabetes Prevention Study (DPS) (16) and the Diabetes Prevention Program (DPP) (17). A comprehensive structured program that targeted dietary modification with a low-calorie, low-fat, low-saturated fat, high-fibre diet and moderate-intensity physical activity of at least 150 minutes per week resulted in a moderate weight loss of approximately 5% of initial body weight. In both studies, the risk reduction for diabetes was 58% at 4 years. On the basis of the observed benefits of healthy behaviour interventions in the DPP, all participants were offered further lifestyle interventions for a median of 5.7 more years, and benefits were sustained for up to 10 years in the Diabetes Prevention Program Outcomes Study (DPPOS) (18). In a follow-up analysis of the DPP intensive lifestyle intervention cohort, 2-year weight loss was the strongest predictor of reduced diabetes incidence (19). Weight cycling, defined as the number of 2.25 kg weight cycles, was positively associated with incident diabetes. After adjustment for baseline weight, the effect of weight cycling remained statistically significant for diabetes risk (19). In another follow up of the DPP study, lower weight and plasma glucose level early on at 6 and 12 months strongly predicted lower subsequent diabetes risk with healthy behaviour interventions although the study was not completely blinded (20). In the long-term follow up of the randomized Finnish Diabetes Prevention Study (DPS), similar results were noted over 13 years with respect to decreased incidence in diabetes (20,21).
In another healthy behaviour intervention trial, 458 Japanese males with IGT were randomly assigned in a 4:1 ratio to a standard intervention (n=356) or an intensive intervention (n=102) and followed for 4 years (22). Intensive treatment was associated with a 67.4% reduction in risk of diabetes (p<0.001). In a more recent trial, 641 Japanese men (aged 30 to 60 years) with overweight and IFG were randomized to either a frequent intervention group (n=311) or a control group (n=330) for 36 months. The frequent intervention group received individual instruction and follow-up support for healthy behaviour interventions from medical staff 9 times. The control group received similar individual instruction 4 times at 12-month intervals during the same period. Results showed an incidence of type 2 diabetes of 12.2% in the frequent intervention group and 16.6% in the control group, with an adjusted hazard ratio (HR) in the frequent intervention group of 0.56 [95% confidence interval (CI) 0.36–0.87]. Post hoc subgroup analyses showed the HR reduced to 0.41 (95% CI 0.24–0.69) among participants with IGT at baseline and to 0.24 (95% CI 0.12–0.48) among those with a baseline A1C level >5.6% (23).
A 23-year follow up of the Chinese Da Qing Diabetes Prevention Trial showed that after 6 years of active healthy behaviour interventions vs. no treatment, the active group had less diabetes, CV and all-cause mortality. This study enrolled 577 people, 439 of whom were assigned to the intervention group and 138 who were assigned to the control group. A total of 174 participants died during the 23 years of follow up (121 in the intervention group vs. 53 in the control group). Cumulative incidence of CVD mortality was 11.9% (95% CI 8.8–15.0) in the intervention group vs. 19.6% (95% Cl 12.9–26.3) in the control group (HR 0.59, 95% CI 0.36–0.96; p=0.033). All-cause mortality was 28.1% (95% CI 23.9–32.4) vs. 38.4% (HR 0.71, 95% CI 0.51–0.99, p=0.049). Incidence of diabetes was 72.6% vs. 89.9% (HR 0.55, 95% CI 0.40–0.76, p=0.001) (24).
Medical Nutrition Therapy
Nutrition therapy and counselling are essential components of the treatment and management of prediabetes. A prospective randomized parallel group study of 76 adults with IFG (or an A1C of 5.7% to 6.4%) found that individualized medical nutrition therapy (MNT) provided by a registered dietitian significantly decreased A1C in individuals diagnosed with prediabetes, compared with usual care after 12 weeks (5.79% vs. 6.01%) (25). The 12-week intervention consisted of four nutrition visits; self-management training; instruction on a high-carbohydrate (60% to 70% daily calories), high-fibre, low-fat (<7% calories from saturated fat) diet; and weight loss (individualized caloric goals to achieve 0.45 to 0.9 kg/week weight loss to achieve 5% body weight loss).
Dietary Patterns
There is strong evidence to support the use of the Mediterranean diet in diabetes prevention. In 2015, Esposito et al conducted a systematic review of all meta-analyses and randomized controlled trials that compared the Mediterranean diet with a control diet for the treatment of type 2 diabetes and prediabetes. Higher adherence to the Mediterranean diet reduced the risk of future diabetes by 19% to 23% (26). Included in this systematic review is one long-term randomized controlled trial, the PREDIMED trial, in which a subgroup analysis restricted to those without diabetes at baseline found that a Mediterranean diet significantly reduced development of type 2 diabetes during follow up (27). Older individuals (55 to 75 years of age) living in Spain with high risk of CVD were randomized to 1 of 3 interventions: Mediterranean diet supplemented with extra virgin olive oil (EVOO) (50 mL/day), Mediterranean diet supplemented with mixed nuts (30 g/day) or a control diet consisting of advice to reduce intake of all types of fat. After a median 4.8-year follow up, a statistically significant 40% relative risk reduction and a non-significant 18% risk reduction in diabetes risk was seen in the Mediterranean diet groups supplemented with EVOO and mixed nuts, respectively, in comparison with the control group. The beneficial effect was attributed to the overall composition of the dietary pattern, and not to calorie restriction, increased physical activity or weight loss because these healthy behaviour interventions were not part of the intervention and between-group changes were negligible.
In addition to the Mediterranean diet, a significant reduction of type 2 diabetes has also been found to be associated with healthy dietary patterns, including the DASH (Dietary Approaches to Stop Hypertension) diet, the AHEI (Alternate Healthy Eating Index) and various other healthy dietary patterns, derived by factor or cluster analysis (28). A meta-analysis of 18 prospective studies from 20 cohorts in four world regions demonstrated that adherence to these healthy diets are consistently associated with a 20% reduced risk of future type 2 diabetes (28). While the nature of diets associated with prevention of type 2 diabetes may vary, these healthy diets share several common components, including whole grains, fruit, vegetables, nuts, legumes, olive oil, white meat/seafood, little or moderate alcohol, reduced intake of red and processed meats and sugar-sweetened beverages.
Diets Emphasizing Specific Foods
Increased consumption of whole grains and dairy products have shown promising results with respect to decreased incidence of type 2 diabetes.
Whole grains
A large prospective cohort of postmenopausal women from the Women's Health Initiative Observational Study demonstrated that the consumption of whole grains was inversely associated with incident type 2 diabetes over a median 7.9 years of follow up (29). Adjusted for age and energy intake per day, successively increasing categories of whole grain consumption were associated with significant reduced risk of developing type 2 diabetes. Women who consumed greater than 2 servings of whole grains per day had a 43% reduced risk of incident type 2 diabetes compared with women who consumed no whole grain (29).
Dairy
A meta-analysis of 17 cohort studies (30) reported an inverse association between intakes of total dairy, low-fat dairy products and cheese and risk of type 2 diabetes (30). Nonlinear inverse associations were observed for total dairy products and yogurt, with most of the benefit being observed when increasing the intake of total dairy products from little to no dairy up to 300 to 400 g/day or yogurt up to 120 to 140 g/day, above which there was no further benefit. The associations between low-fat dairy products and cheese and type 2 diabetes were borderline nonlinear (p≤0.06), with most of the benefit observed when increasing the intake of these items up to 300 to 400 g/day for low-fat dairy, and up to ~50 g/day for cheese.
Physical Activity
Higher levels of leisure time physical activity (LTPA) are associated with substantially lower incidence of type 2 diabetes (31). A systematic review and dose-response meta-analysis which included over one million individuals from 28 prospective cohort studies provided information on the association between LTPA (24 cohorts) or total physical activity (4 cohorts) and incidence of type 2 diabetes (31). The results suggested a curvilinear relationship and found a risk reduction of 26% for type 2 diabetes (31) among those who achieved 11.25 metabolic equivalents (MET) h/week (equivalent to 150 minutes per week of moderate activity). Individuals who attained twice this amount of physical activity were associated with a risk reduction of 36%, with even further risk reductions, 53%, at a higher dose of 60 MET/week. The greatest relative benefits were attained at low levels of activity, but further benefits can be recognized at levels that go well beyond those prescribed by the current minimum recommendation of 150 minutes per week of moderate intense activity. Similarly, the 25-year cohort Coronary Artery Risk Development in Young Adults (CARDIA) study measured fitness in 4,373 participants from young adulthood to middle age and found that fitness was associated with a lower risk for developing prediabetes and type 2 diabetes, even when adjusting for body mass index (BMI) over this time period (32). Future research is needed to consider the dose-response relationship of physical activity and type 2 diabetes prevention in ethnically diverse populations.
Pharmacotherapy
Metformin
Metformin was used in a second randomized arm of the DPP and compared to lifestyle and to placebo (17). A dosage of 850 mg twice daily for an average of 2.8 years significantly decreased progression to diabetes by 31% compared to placebo. An analysis of the subgroup with FPG 6.1 to 6.9 mmol/L showed a 48% reduction in diabetes diagnosis. In the DPP population, metformin did not have a significant effect in the older age group (>60 years) and in subjects with less obesity (BMI <35 kg/m
A subsequent analysis of DPP that analyzed diabetes incidence defined by A1C ≥6.5% found a 44% reduction by metformin and 49% by healthy behaviour interventions during the DPP, and by 38% by metformin and 29% by healthy behaviour interventions over 10 years of follow up (35). Unlike the primary DPP and DPPOS findings based on glucose criteria, metformin and healthy behaviour interventions were similarly effective in preventing diabetes defined by A1C. Additionally, there was a significant interaction (p<0.01) between baseline A1C and the effects of healthy behaviour interventions and metformin treatment were greater at higher baseline A1C between 6.0% to 6.4% range, compared to lower A1C baseline categories.
Overall, metformin may be considered as a strategy to prevent type 2 diabetes in people with IGT (especially in combination with IFG or with elevated A1C between 6.0% to 6.4% range). Metformin may be more effective among younger individuals (<60 years) with significant obesity (>35 kg/m
Thiazolidinediones
The Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication (DREAM) trial randomized 5,269 people with IGT and/or IFG, in a 2 x 2 factorial fashion, to ramipril (up to 15 mg/ day) and/or rosiglitazone (8 mg/day) vs. placebo (36,37). Eligible subjects were >30 years of age and not known to have CVD. The primary outcome of DREAM was a composite of development of diabetes or death. Treatment with rosiglitazone resulted in a 60% reduction in the primary composite outcome of diabetes or death (HR 0.40, 95% CI 0.35–0.46), primarily due to a 62% relative reduction in the risk of progression to diabetes (HR 0.38, 95% Cl 0.33–0.44). In the Actos Now for the Prevention of Diabetes (ACT NOW) study, 602 high-risk participants with IGT were randomized to receive pioglitazone or placebo and were followed for 2.4 years. Pioglitazone decreased the conversion of IGT to type 2 diabetes by 72% (p<0.00001) (38). In the CAnadian Normoglycaemia Outcomes Evaluation (CANOE) trial, the combination of metformin 500 mg twice daily and rosiglitazone 2 mg twice daily was found to reduce the progression to diabetes by 66% (95% CI 41–80) among 103 people with IGT compared to 104 people randomized to placebo over a median of 3.9 years (39).
Recently, the Insulin Resistance Intervention after Stroke (IRIS) trial demonstrated that pioglitazone reduced the development of type 2 diabetes by 52% over 4.8 years along with also reducing stroke and myocardial infarction (MI) after a recent ischemic stroke or transient ischemic attack (TIA) in people with insulin resistance and prediabetes (40). A total of 3,876 people with recent ischemic stroke or TIA, no history of diabetes, FPG <7.0 mmol/L and insulin resistance by homeostasis model assessment of insulin resistance (HOMA-IR) score >3.0 were randomly assigned to pioglitazone or placebo. Surveillance for diabetes onset during the trial was accomplished by periodic interviews and annual FPG testing. At baseline, the mean FPG, A1C, insulin and HOMA-IR were 5.46 mmol/L, 5.8%, 22.4 mIU/mL, and 5.4, respectively. After 1 year, mean HOMA-IR and FPG decreased to 4.1 and 5.3 mmol/L in the pioglitazone group and rose to 5.7 and 5.5 mmol/L in the placebo group (all p<0.0001). Over a median follow up of 4.8 years, diabetes developed in 73 (3.8%) participants assigned to pioglitazone compared with 149 (7.7%) assigned to placebo (HR 0.48, 95% CI 0.33–0.69, p<0.0001). This effect was predominantly driven by those with initial IFG (FPG >5.6 mmol/L; HR 0.41, 95% CI 0.30–0.57) or elevated A1C (>5.7%, HR 0.46, 95% Cl 0.34–0.62]). The study did not provide information whether this effect would be sustained. Other limitations include reduction in type 2 diabetes not being the primary outcome measure, poor adherence, no washout of study drug and some people likely already had diabetes at study entry.
Despite the favourable effects of thiazolidinediones on delaying the development of type 2 diabetes, the multiple potential adverse effects and warnings in this class of medication make it difficult to recommend their widespread use in people with IFG or IGT.
Acarbose
The Study to Prevent Non-Insulin Dependent Diabetes (STOP- NIDDM) used acarbose at a dosage of 100 mg three times a day in a 5-year study with a mean follow up of 3.3 years (41). Overall, there was a 25% reduction in the risk of progression to diabetes when the diagnosis was based on one OGTT and a 36% reduction in the risk of progression to diabetes when the diagnosis was based on two consecutive OGTTs. However, when the acarbose was discontinued, the effect did not persist (41). In another trial, 1,780 Japanese people with IGT were randomly assigned to oral voglibose 0.2 mg three times a day (n=897) or placebo (n=883) (42). Results showed that, over a mean of 48.1 weeks, voglibose was more effective than placebo at reducing the progression to type 2 diabetes (5.6% vs. 11.9%, HR 0.595, 95% CI 0.433–0.818, p=0.0014). More subjects in the voglibose group achieved normoglycemia than in the placebo group (66.8% vs. 51.5%, HR 1.539, 95% Cl 1.357–1.746, p<0.0001).
Orlistat
The Xenical in the Prevention of Diabetes in Obese Subjects (XENDOS) study examined the effect of orlistat in combination with an intensive lifestyle modification program (diet and exercise) on the prevention of diabetes in 3,305 individuals with obesity (43). Subjects were randomized to orlistat 120 mg or placebo three times a day with meals for 4 years. Weight loss was observed in both groups, but the orlistat group lost significantly more (5.8 vs. 3 kg, p<0.001). Compared to placebo, orlistat treatment was associated with a further 37% reduction in the incidence of diabetes. However, two important methodological limitations affect the interpretation of these results. First, there was a very high dropout rate of 48% in the orlistat group and 66% in the placebo group. Second, the last observation carried forward was used for analysis, which is generally not favoured for prevention or survival studies.
Liraglutide
Liraglutide has been shown to prevent IGT conversion to type 2 diabetes and cause reversion to normoglycemia (44). In a 20-week study, liraglutide was administered to 564 individuals with obesity who did not have diabetes, 31% of whom had IGT. Subjects were randomized to 1 of 4 liraglutide doses (1.2 mg, 1.8 mg, 2.4 mg or 3.0 mg, n=90–95) or to placebo (n=98), or to orlistat (120 mg, n=95) three times daily. A1C was reduced by 0.14% to 0.24%. The prevalence of prediabetes decreased by 84% to 96% with liraglutide 1.8 mg, 2.4 mg and 3.0 mg doses. In a secondary outcome analysis from another randomized trial of 56 weeks duration among 3,731 participants with obesity who did not have type 2 diabetes (61% participants with IGT and remaining participants with normoglycemia at baseline), 4 participants in the liraglutide 3.0 mg group and 14 in the placebo group developed diabetes (p<0.01) (45).
Recently, in a 3-year extension study of the Satiety and Clinical Adiposity — Liraglutide Evidence in Nondiabetic and Diabetic Individuals (SCALE) Obesity and Prediabetes study, adults with prediabetes and a body mass index of at least 30 kg/m
Vitamin D
A systematic review and meta-analysis compared vitamin D3 supplementation with placebo or a non-vitamin D supplement in adults with normal glucose tolerance, prediabetes, or type 2 diabetes (47). Thirty-five trials (43,407 participants) with variable risk of bias were included. Vitamin D had no significant effects on insulin resistance [homeostasis model assessment of insulin resistance: MD −0.04; 95% Cl -0.30 to 0.22, I-squared statistic (I
Bariatric Surgery
A systematic review and meta-analysis consisting of 18 studies (43,669 participants, 30,774 with IGT and/or IFG), looking at people with obesity at risk for type 2 diabetes (BMI >30 kg/m
Diabetes Prevention in High-Risk Ethnicities
Certain ethnic groups, including African, Arab, Asian, Hispanic, Indigenous and South Asian peoples, are at very high risk for and have a high prevalence of type 2 diabetes (12% to 15% in the Western world) (48,49). The reasons for this are multifactorial and include genetic susceptibility, altered fat distribution (more visceral fat with greater insulin resistance) and higher prevalence of metabolic syndrome. Many of them develop diabetes at a younger age and often have complications at the time of diagnosis due to long-standing, pre-existing diabetes. As a result, there may be a benefit of delaying the onset of diabetes in this population. The Indian Diabetes Prevention Programme randomized 531 people with IGT diabetes in Chennai, India to 4 groups: healthy behaviour interventions; metformin; healthy behaviour interventions and metformin; and control with a median follow up of 30 months. Progression to diabetes in the control group was high (55%) over 3 years (50). The relative risk reduction was 28.5% with healthy behaviour interventions, 26.4% with metformin and 28.2% with healthy behaviour interventions and metformin compared with the control group.
Another study utilizing a stepwise approach of healthy behaviour interventions with the option of adding metformin reduced the risk of type 2 diabetes in Asian Indian adults (51). This was a randomized, controlled trial of 578 Asian Indian adults with overweight or obesity with isolated IGT, isolated IFG, or IFG and IGT in Chennai, India. Participants were randomized to standard lifestyle advice (control) or a 6-month, culturally tailored, United States Diabetes Prevention Program-based lifestyle curriculum, plus stepwise addition of metformin (500 mg twice daily) for participants at highest risk of conversion to diabetes at 4+ months of follow up, defined as having either IFG+IGT or IFG and A1C ≥5.7%. The primary outcome of diabetes incidence was assessed biannually and compared across study arms using an intention-to-treat analysis. During 3 years of follow up, 34.9% of control and 25.7% of intervention participants developed diabetes (p=0.014); the relative risk reduction (RRR) was 32% (95% CI 7–50), and the number needed to treat to prevent one case of diabetes was 9.8. The RRR varied by prediabetes type and was only significant for IFG and IGT (RRR =36%), although the magnitude was similar but non-significant for isolated IGT (RRR =31%). Among subgroups, RRR was stronger in participants 50 years or older, male, or with obesity. Most participants (72.0%) required metformin in addition to healthy behaviour interventions, although there was variability by prediabetes type (isolated IFG, 76.5%; IFG and IGT, 83.0%; isolated IGT, 51.3%). Limitations included lack of power for subgroup comparisons, simplistic assessment of physical activity, and potential for lack of generalizability since the population was Asian Indian only.
The above approach of stepwise prevention intervention may lead to cost savings, fewer complications and lower morbidity, but it remains to be proven with hard clinical endpoints. Healthy behaviour interventions not only reduce the risk of diabetes but have other health benefits, so the overall benefit is positive with little harm. One must keep in mind that the measures of prevention must be delivered in a culturally sensitive manner to these populations.
Population Level Interventions for Prevention of Type 2 Diabetes
At a macro-level, the type 2 diabetes epidemic has been attributed to urbanization and environmental transitions, including sedentary occupations, increased mechanization, improved transportation, as well as increased accessibility to unhealthy diets with high-calorie content and large portion sizes. In recent decades, men and women around the globe (and in Canada) have gained weight, largely due to changes in dietary patterns and decreased physical activity levels. The dominant effect of obesity in precipitating glucose intolerance and its consequences suggests that reversal of the diabetes epidemic can only come about with urgent and substantial changes to health behaviours on a population level. It is important to recognize that the health sector on its own cannot accomplish population-wide changes. New strategic relationships with groups that have an impact on health (e.g. food industry and construction industry) are needed to help create an environment more conducive to an active lifestyle and healthy eating habits.
Major legislative and other regulatory measures may be required similar to those needed to address illness arising from tobacco usage. Some examples of this are transformation of work environment, development of school curriculum to improve physical and nutritional education, improvement of food labelling on packaged foods, mandating nutrition labelling of restaurant foods and regulating advertisements, especially to children, etc. In addition, food choices may be influenced by price increases (taxation) or price decreases (subsidies). In a recent systematic review and meta-analysis (52), a 10% price subsidy increased consumption of healthy foods by 12% (95% CI 10±15%), including intake of fruits and vegetables by 14% (95% CI 11±17%); whereas a 10% increase in price decreased consumption of unhealthy foods by 6% (95% CI 4±8%), including sugar-sweetened beverage intake by 7% (95% CI 3±10%). Greater intake of sugar-sweetened beverages has been associated with higher type 2 diabetes risk in a meta-analysis (53) and a pooled analysis of European cohorts (54). This association remains significant even after adjusting for BMI, suggesting that the deleterious effects of sugar-sweetened beverages on diabetes are not entirely mediated by body weight. Diabetes Canada has a public health advocacy campaign recommending (i) limited intake of free sugars to <10% of total daily calorie intake, and (ii) limited intake of sugar-sweetened beverages.
Recommendations
- In individuals with prediabetes, a structured program of healthy behaviour interventions that includes moderate weight loss and regular physical activity of a minimum of 150 minutes per week over 5 days a week should be implemented to reduce the risk of type 2 diabetes [Grade A, Level 1A (16,17) for individuals with IGT; Grade B, Level 2 [23] for individuals with IFG; Grade D, Consensus for individuals with A1C 6.0%–6.4%].
- In individuals at risk for type 2 diabetes, dietary patterns may be used to reduce the risk of diabetes, specifically:
- In individuals with prediabetes, pharmacologic therapy with metformin may be used to reduce the risk of type 2 diabetes [Grade A, Level 1A (17,33) for individuals with IGT; Grade D, Consensus for individuals with IFG or A1C 6.0%–6.4%].
Abbreviations:
A1C, glycated hemoglobin; BMI, body mass index; CI, confidence interval; CV, cardiovascular; CVD, cardiovascular disease; EVOO, extra virgin olive oil; GDM, gestational diabetes; HR, hazard ratio; IFG, impaired fasting glucose; IGT, impaired glucose tolerance.
Literature Review Flow Diagram for Chapter 5: Reducing the Risk of Developing Diabetes
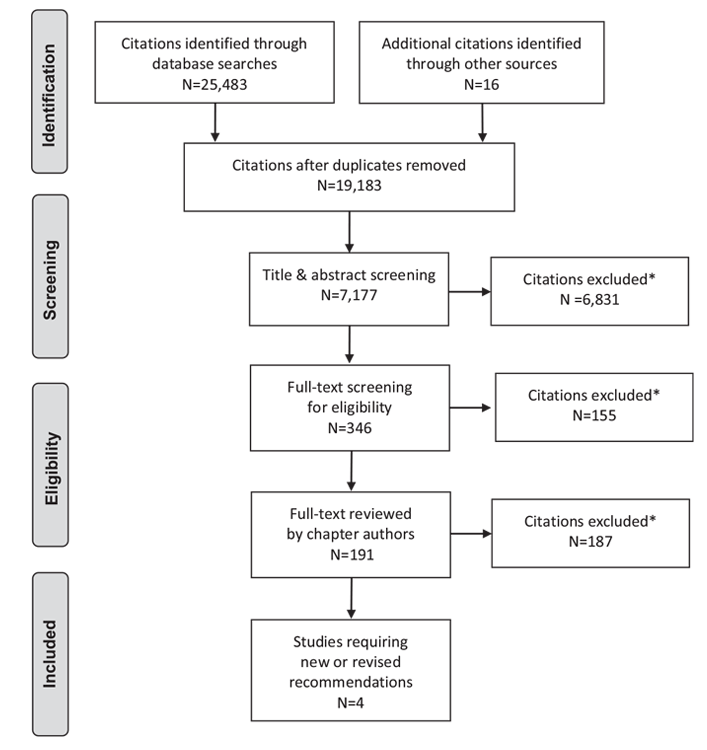
*Excluded based on: population, intervention/exposure, comparator/control or study design
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi:10.1371/journal.pmed1000097 (55).
For more information, visit www.prisma-statement.org.
Author Disclosures
Dr. Prebtani reports support from Novo Nordisk, Eli Lilly, Boehringer Ingelheim, Sanofi and Janssen, outside the submitted work. Dr. Bajaj reports personal fees from Abbott, and grants and personal fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, and Sanofi, outside the submitted work. Dr. Goldenberg reports personal fees from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, Sanofi, and Servier, outside the submitted work. Yvonne Mullan has nothing to disclose.
References
- Gale EA, Bingley PJ, Emmett CL, et al. European Nicotinamide Diabetes Intervention Trial (ENDIT): A randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet 2004;363:925–31.
- Diabetes Prevention Trial–Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002;346:1685–91.
- Skyler JS, Krischer JP,Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial–Type 1. Diabetes Care 2005;28:1068–76.
- Knip M, Akerblom HK, Becker D, et al. Hydrolyzed infant formula and early betacell autoimmunity: A randomized clinical trial. JAMA 2014;311:2279–87.
- Näntö-Salonen K, Kupila A, Simell S, et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: A double-blind, randomised controlled trial. Lancet 2008;372:1746–55.
- Alberti KG, Zimmet P, Shaw J. International Diabetes Federation: A consensus on type 2 diabetes prevention. Diabet Med 2007;24:451–63.
- Narayan KM, Thompson TJ, Boyle JP, et al. The use of population attributable risk to estimate the impact of prevention and early detection of type 2 diabetes on population-wide mortality risk in US males. Health Care Manag Sci 1999;2:223–7.
- American Diabetes Association. Economic costs of diabetes in the U.S. In 2007. Diabetes Care 2008;31:596–615.
- Micucci S, Thomas H, Vohra J. The effectiveness of school-based strategies for the primary prevention of obesity and for promoting physical activity and/or nutrition, the major modifiable risk factors for type 2 diabetes: A review of reviews. Hamilton: Effective Public Health Practice Project, 2002. https://www.healthevidence.org/view-article.aspx?a=effectiveness-school-basedstrategies-primary-prevention-obesity-promoting-16147.
- Daniel M, Green LW, Marion SA, et al. Effectiveness of community-directed diabetes prevention and control in a rural Aboriginal population in British Columbia, Canada. Soc Sci Med 1999;48:815–32.
- Simmons D, Voyle J, Swinburn B, et al. Community-based approaches for the primary prevention of non-insulin-dependent diabetes mellitus. Diabet Med 1997;14:519–26.
- Charles MA, Fontbonne A, Thibult N, et al. Risk factors for NIDDM in white population. Paris prospective study. Diabetes 1991;40:796–9.
- Eastman RC, Cowle CC, Harris MI. Undiagnosed diabetes or impaired glucose tolerance and cardiovascular risk. Diabetes Care 2002;20:127–8.
- Tuomilehto J, Knowler WC, Zimmet P. Primary prevention of non-insulindependent diabetes mellitus. Diabetes Metab Rev 1992;8:339–53.
- Sumamo Schellenberg E, Dryden DM, Vandermeer B, et al. Lifestyle interventions for patients with and at risk for type 2 diabetes: A systematic review and meta-analysis. Ann Intern Med 2013;159:543–51.
- Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance.N Engl J Med 2001;344:1343–50.
- Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403.
- Diabetes Prevention Program Research Group, Knowler WC, Fowler SE, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–86.
- Delahanty LM, Pan Q, Jablonski KA, et al. Effects of weight loss, weight cycling, and weight loss maintenance on diabetes incidence and change in cardiometabolic traits in the Diabetes Prevention Program. Diabetes Care 2014;37:2738–45.
- Maruthur NM, Ma Y, Delahanty LM, et al. Early response to preventive strategies in the Diabetes Prevention Program. J Gen Intern Med 2013;28:1629–36.
- Lindstrom J, Peltonen M, Eriksson JG, et al. Improved lifestyle and decreased diabetes risk over 13 years: Long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetologia 2013;56:284–93.
- Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: A Japanese trial in IGT males. Diabetes Res Clin Pract 2005;67:152–62.
- Saito T, Watanabe M, Nishida J, et al. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: A randomized controlled trial. Arch Intern Med 2011;171:1352–60.
- Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: A 23-year follow-up study.Lancet Diabetes Endocrinol 2014;2:474–80.
- Parker AR, Byham-Gray L, Denmark R, et al. The effect of medical nutrition therapy by a registered dietitian nutritionist in patients with prediabetes participating in a randomized controlled clinical research trial. J Acad Nutr Diet 2014;114:1739–48.
- Esposito K, Maiorino MI, Bellastella G, et al. A journey into a Mediterranean diet and type 2 diabetes: A systematic review with meta-analyses. BMJ Open 2015;5:e008222.
- Martinez-Gonzalez MA, Salas-Salvado J, Estruch R, et al. Benefits of the Mediterranean diet: Insights from the PREDIMED Study. Prog Cardiovasc Dis 2015;58:50–60.
- Esposito K, Chiodini P, Maiorino MI, et al. Which diet for prevention of type 2 diabetes? A meta-analysis of prospective studies. Endocrine 2014;47:107–16.
- Parker ED, Liu S, Van Horn L, et al. The association of whole grain consumption with incident type 2 diabetes: The Women’s Health Initiative Observational Study. Ann Epidemiol 2013;23:321–7.
- Aune D, Norat T, Romundstad P, et al. Dairy products and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr 2013;98:1066–83.
- Smith AD, Crippa A,Woodcock J, et al. Physical activity and incident type 2 diabetes mellitus: A systematic review and dose-response meta-analysis of prospective cohort studies. Diabetologia 2016;59:2527–45.
- Chow LS, Odegaard AO, Bosch TA, et al. Twenty year fitness trends in young adults and incidence of prediabetes and diabetes: The CARDIA study. Diabetologia 2016;59:1659–65.
- Aroda VR, Christophi CA, Edelstein SL, et al. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: The Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab 2015;100:1646–53.
- The Diabetes Prevention Program Research Group. Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program. Diabetes Care 2003;26:977–80.
- Diabetes Prevention Program Research G. HbA1c as a predictor of diabetes and as an outcome in the diabetes prevention program: A randomized clinical trial. Diabetes Care 2015;38:51–8.
- DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators, Gerstein HC, Yusuf S, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet 2006;368:1096–105.
- DREAM Trial Investigators, Bosch J, Yusuf S, et al. Effect of ramipril on the incidence of diabetes. N Engl J Med 2006;355:1551–62.
- DeFronzo RA, Tripathy D, Schwenke DC, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. New Engl J Med 2011;364:1104-15.
- Zinman B, Harris SB, Neuman J, et al. Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): A double-blind randomised controlled study. Lancet 2010;376:103–11.
- Inzucchi SE, Viscoli CM, Young LH, et al. Pioglitazone prevents diabetes in patients with insulin resistance and cerebrovascular disease. Diabetes Care 2016;39:1684–92.
- Chiasson JL, Josse RG, Gomis R, et al. Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomised trial. Lancet 2002;359:2072–7.
- Kawamori R, Tajima N, Iwamoto Y, et al. Voglibose for prevention of type 2 diabetes mellitus: A randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet 2009;373:1607–14.
- Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of diabetes in obese subjects (XENDOS) study: A randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27:155–61.
- Astrup A, Rossner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: A randomised, double-blind, placebo-controlled study. Lancet 2009;374:1606–16.
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015;373:11–22.
- le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: A randomised, double-blind trial. Lancet 2017;389:1399–409.
- Seida JC, Mitri J, Colmers IN, et al. Clinical review: Effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: A systematic review and meta-analysis. J Clin Endocrinol Metab 2014;99:3551–60.
- Chowdhury TA, Grace C, Kopelman PG. Preventing diabetes in South Asians: Too little action and too late. BMJ 2003;327:1059–60.
- Egede LE, Dagogo-Jack S. Epidemiology of type 2 diabetes: Focus on ethnic minorities. Med Clin North Am 2005;89:949–75, viii.
- Ramachandran A, Snehalatha C, Mary S, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–97.
- Weber MB, Ranjani H, Staimez LR, et al. The stepwise approach to diabetes prevention: Results from the D-CLIP randomized controlled trial. Diabetes Care 2016;39:1760–7.
- Afshin A, Penalvo JL, Del Gobbo L, et al. The prospective impact of food pricing on improving dietary consumption: A systematic review and meta-analysis. PLoS ONE 2017;12:e0172277.
- Wang M, Yu M, Fang L, et al. Association between sugar-sweetened beverages and type 2 diabetes: A meta-analysis. J Diabetes Investig 2015;6:360–6.
- Romaguera D, Norat T,Wark PA, et al. Consumption of sweet beverages and type 2 diabetes incidence in European adults: Results from EPIC-InterAct. Diabetologia 2013;56:1520–30.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 2009;6:e1000097.
*The Canadian Diabetes Association is the registered owner of the name Diabetes Canada. All content on guidelines.diabetes.ca, CPG Apps and in our online store remains exactly the same. For questions, contact communications@diabetes.ca.


