Chapter Headings
We acknowledge that a position statement about DIY AID is only possible because of the pioneering work of individuals living with diabetes, and their loved ones, who founded the #WeAreNotWaiting movement by believing in the power of possibility and being willing to pay it forward. Their work has been instrumental in propelling the field of automated insulin delivery forward.
Introduction—Why Is This Position Paper Needed?
Type 1 diabetes (T1D) is a complex chronic condition characterized by a complete lack of endogenous insulin production. Despite advances in insulin analogues and glucose monitoring systems, living with T1D remains burdensome [1]. Automated insulin delivery (AID) systems have been proven in randomized trials to help reach glycemic targets while reducing the burden of self-management for people with T1D and their caregivers (PWD ∗). AID systems rely on a computer-based algorithm to modulate insulin delivery by an insulin pump based on glucose levels derived from real-time continuous glucose monitoring (rtCGM) data. This real-time adjustment of insulin delivery has been shown to ease burden and increase the safety of living with T1D, and so the American Diabetes Association (ADA) has recently added a standard of care statement that commercially approved AID should be offered to youth and adults with TID who can use the devices safely [2].
Clinical use of AID systems was pioneered by members of the T1D community who developed their own open-source AID systems available since 2014, known widely as do-it-yourself (DIY) AID or “looping”. Commercial systems, available since 2016, have more recently been approved by Health Canada and reached the Canadian market, but access varies by province. The term “open source” describes the open publishing and sharing of the computer code and algorithms which are constantly being upgraded and improved by the T1D developer community. Recently, an international consensus paper was published [3] using the term “open-source AID”; however, this Diabetes Canada Expert Working Group has opted to use the term DIY AID to emphasize the very active role the user has to take to build and maintain their own system from the open-source code and its related programs, while striving to maximize safety and effectiveness to achieve the individual user’s goals, which often include decreased glycemic variability and decreased burden of living with diabetes [4].
Despite the increasing popularity and compelling testimonials of DIY AID in the T1D community, diabetes health-care practitioners (HCPs) have limited knowledge of DIY AID and are hesitant to discuss or care for people using these systems given the lack of regulatory approval and poor understanding of how they work [5]. In some other countries, guidelines advise against providers prescribing DIY AID systems, but recommend that providers assist in diabetes management to ensure individual safety [2,6]. These uncertainties have been examined in the Canadian context [7] and Canadian HCPs identified national guidance as important to help them better deliver care to people with T1D.
Diabetes Canada has long advocated individualized targets for PWD and the importance of autonomy [8]. Thus, the desires and wishes of PWD should be sought and their preferences be central in shared decision making regarding their diabetes management. PWD should be able to work with their care team to find the solution that best meets their needs to deliver optimal glycemic outcomes and reduce the risk of acute and chronic complications, with an acceptable and sustainable day-to-day burden of living with T1D. This Diabetes Canada position statement and the user’s guide (see page 389) seek to provide guidance for Canadian HCPs to support individuals using non-regulated technologies having carefully considered the evidence base, as well as the legal and ethical issues in this novel area. This paper includes guidance on the use of DIY AID systems, including Loop, OpenAPS, and AndroidAPS, which had supporting evidence available at the time of publication.
Methods
A diverse group of experts in diabetes technology was convened by the Diabetes Canada Clinical Practice Guidelines (CPG) Steering Committee to determine the scope and purpose of the position statement. Members included HCPs caring for people with T1D using DIY AID (I.J.H., A.E.M., P.S.), individuals with lived experience with T1D or as a parent/caregiver to a child with T1D (A.C., H.O.W., K.F., L.C.), individuals with experience using DIY AID (A.C., H.O.W., K.F.), and individuals with legal (L.C.) and ethics (U.S.) expertise. A previous scoping review [9] completed as part of a master's thesis on DIY AID by 1 author (A.E.M.) formed the basis for our search strategy. The initial literature review included all publications to December 31, 2021. Relevant articles published in English were systematically sought using the databases Embase, Medline, Web of Science, Scopus, Proquest, and Cochrane Library. For this position statement, the literature review was extended to June 30, 2022, using the same search strategy and including a search of the conference syllabus of the ADA, Advanced Technology and Therapeutics in Diabetes (ATTD), and Diabetes UK conferences for relevant abstracts, and found an additional 19 relevant publications, which were reviewed by members of the working group.
What Do We Know About DIY AID
HCP perspectives
There have been a number of quantitative and qualitative studies examining HCPs’ comfort with supporting PWD who choose to use DIY AID [5,10,11]. Morrison et al surveyed 204 Canadian HCPs (33% registered dietitians [RDs], 32% registered nurses [RNs] 28% endocrinologists) in June 2021 [5]. Respondents expressed greater experience with commercial systems (median 6 to 24 users per practice), relative to DIY AID systems (1 to 5 users), in practices with a median of 100 to 500 individuals with T1D. Comfort levels with these technologies were commensurate with experience; 73% were comfortable supporting commercial systems, relative to just 22% with DIY AID systems. Specific barriers highlighted to DIY system use included lack of exposure, lack of high-quality published data, and medico-legal concerns. Both HCP and user education, in addition to clinical practice guidelines, were felt to be required in order to improve confidence in recommending AID use to individuals in their practice [5].
Another often cited barrier is the lack of regulatory approval for DIY AID, exacerbated by the ADA standards of care [2] and US Food and Drug Administration (FDA) warnings against the use of such technology [12]. However, as presented by the ATTD workshop on DIY AID in 2020, T1D is inherently a DIY condition, and all therapies, whether approved or not, require the user to make real-time decisions that come with inherent risk. Diabetes Canada supports autonomy, and self efficacy is a key skill that is further enhanced when individuals (or families) choose DIY AID. The peer mentoring inherent to the online community provides much more frequent touch points and support for users than traditional models of care where HCP visits occur every few months. The workshop concluded that there is a risk that PWD will stop attending care altogether if their HCPs do not support the use of DIY AID [12].
Some clinicians may feel threatened by the role reversal where the PWD is the expert in DIY AID, as described by Dinneen and McMorrow in “The expert patient will see u now, Doctor” [13]. Using DIY AID as a model, they describe how the dynamic needs to shift away from the physician expert towards a long-term therapeutic relationship focused on meeting the individual’s goals. Further, clinicians have biases regarding whom they think are best suited for AID, with educational level/cognitive ability as the most prominent factor [5]. Strikingly, in a separate study, HCP views changed when interviewed after 6 months' experience in supporting PWD using a commercial closed-loop system. Educated, technologically competent individuals tended to over-interact with the system. It was individuals who HCPs had assumed would struggle to understand and use the technology who seemed to benefit more by allowing the system to operate without interference. These observations led to the conclusion that all individuals should be given the chance to try AID [14]. Clinicians need to be conscious of their assumptions and biases that may result in inappropriate gatekeeping, thus preventing PWD from accessing helpful technologies. We suggest that the evidence and experience to date indicate that clinicians should discuss AID, including both commercial and DIY options, with all people living with T1D.
Individual perspectives
DIY AID systems were created by passionate and technologically adept individuals with lived experience out of a deep personal need for more effective and less burdensome solutions for safe glucose management. Since no commercial AID systems were available until 2016, PWD and caregivers used their skills to create DIY AID systems and made them publicly available for free; technology built for the diabetes community by the diabetes community. Current opinion is that DIY systems can add some degree of individualization of parameters compared to regulatory-approved systems. The nature of open-source insulin delivery systems is that the developers rapidly iterate adding new “branches” of code that allow for improvements in the system much faster than commercial AID systems, which require regulatory approval prior to making changes to algorithms and features.
DIY AID systems have become increasingly popular, with more than 9,000 users worldwide [10]. Although there are no precise estimates of how many DIY AID users live in Canada, there are 2,575 Canadians in the Looped Facebook group (an online community-driven support group comprising PWD, caregivers, HCPs, and researchers) [15]. The popularity of DIY AID in Canada may, in part, be due to the large number of PWD who do not have access to HCPs with expertise in T1D or private or public insurance coverage for commercial AID systems. The online DIY AID community has filled some of these gaps by acting as a supportive resource for PWD not only to troubleshoot the technology, but also in managing T1D in general—a recurrent theme identified in qualitative analyses [16].
According to respondents of a 2021 survey of 662 DIY AID users (99 from Canada), people choosing DIY AID do so for the following reasons: transparency (they want to see how the system works), interoperability (they want options with regards to the device, algorithm, and continuous glucose monitoring [CGM]), desire for open-source software, limited commercial options available or accessible, customization (including the ability to set lower blood glucose targets compared to commercial systems), user-led design features, and the ability for DIY systems to iterate faster than their commercial counterparts [17,18].
DIY AID users report feeling empowered by the ability to customize their systems and from the greater understanding of their diabetes and the physiology they have achieved by building their system [10]. Parents and caregivers share that DIY AID systems provide improved quality of life, sleep quality, and glycemic management, all of which were motivating factors for using DIY AID [19] (Textbox 1). Typically, DIY AID users have been highly educated, with access to disposable income, and have a high level of computer competency [17,18]. The major barriers to building DIY AID systems reported by parents and caregivers are their limited technical skills, lack of support from HCPs, and a perceived inability to maintain DIY AID systems [20]. Discontinuation of DIY AID systems is often because of difficulty fine-tuning settings, which could be mitigated by improved HCP education and support [21].
Textbox 1. Choosing DIY over commercial AID systems
- Quotes from people using the systems and why they choose to do so over commercial systems [17]:
- “Better blood glucose control than commercial systems. Better integration with my phone and Apple watch.” (Adult, Male, Canada, Loop)
- “DIY looping is the only automated system that is advanced enough or reliable enough to be useful. DIY is also more customizable, so better suited for me than commercial systems currently available to me.” (Adult, Female, Australia, Loop)
- “Commercial systems aren’t good enough at reducing the burden.” (Adult parent of child, Female, United States, Loop)
- “The closed-loop DIY system is more flexible than commercial systems. The authors of these systems test them on themselves, on their family members and, therefore, create these systems so that they can actually use them.” (Adult, Male, Slovakia, AndroidAPS)
Efficacy of DIY AID
The often cited HCP concern about the lack of high-quality published data [5] may actually be a constructed rather than a true barrier to use. Multiple retrospective and prospective studies of DIY AID, incorporating more than 55 million hours of real-world data, consistently suggest beneficial outcomes for both glycemia and quality of life, which have now been confirmed in a recently published randomized controlled trial (RCT) [22]. Given the growing evidence base, it is no longer appropriate to cite a lack of evidence as justification for not discussing or supporting DIY AID use.
Glycemia
Improvements in both glycated hemoglobin (A1C) (0.3%–0.9%) and time in range (TIR) (0%–23%) are seen with initiation of DIY AID [4,23–41] in self-report and observational studies (Table 1
Table 1
Changes in A1C (%) and percentage time in range (TIR 3.9e10 mmol/L) with DIY AID use in self-reported and observational studies [4,23-41]
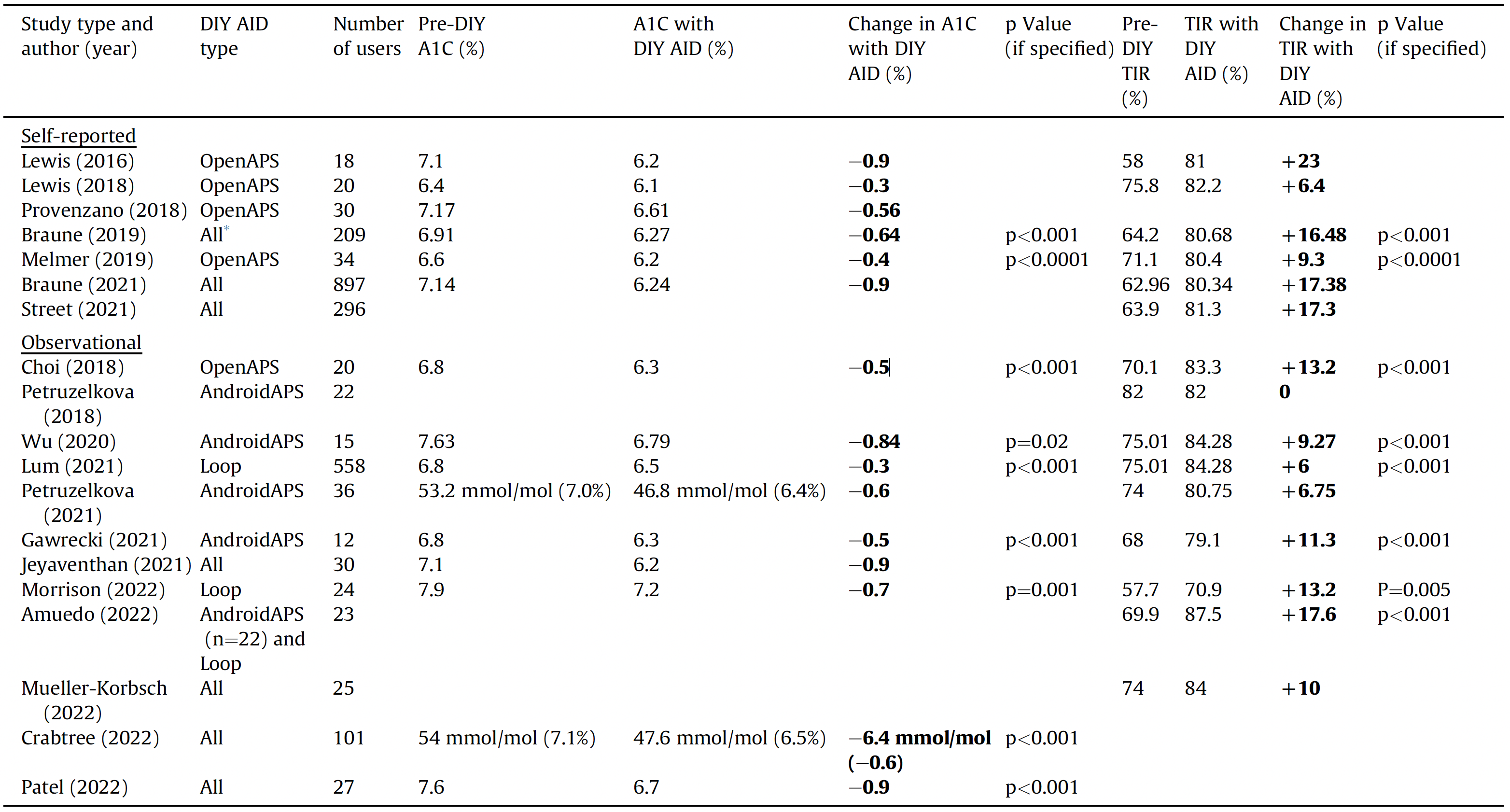
* All refers to Loop, Android APS, and OpenAPS.
A1C, glycated hemoglobin; AID, automated insulin delivery; DIY, do-it-yourself.
Quality of life
Improved quality of life has been observed in multiple studies that have used validated quantitative scores with the initiation of DIY AID. This includes improvements in diabetes-related distress (as measured by the Diabetes Distress Scale) [32,43], fear of hypoglycemia (Hypoglycemia Fear Score) [32,43], and diabetes impact and device satisfaction (DIDS) [4]. A qualitative study of Loop users (n=72) additionally highlighted the substantial impact of DIY systems in decreasing the mental or behavioural burden associated with diabetes management, with notable improvements reported, particularly in overnight glycemia and sleep quality [10].
Table 2
Randomized controlled trial data to demonstrate glycemic outcomes (A1C and TIR 3.9e10 mmol/L) with DIY AID vs SAP [42]
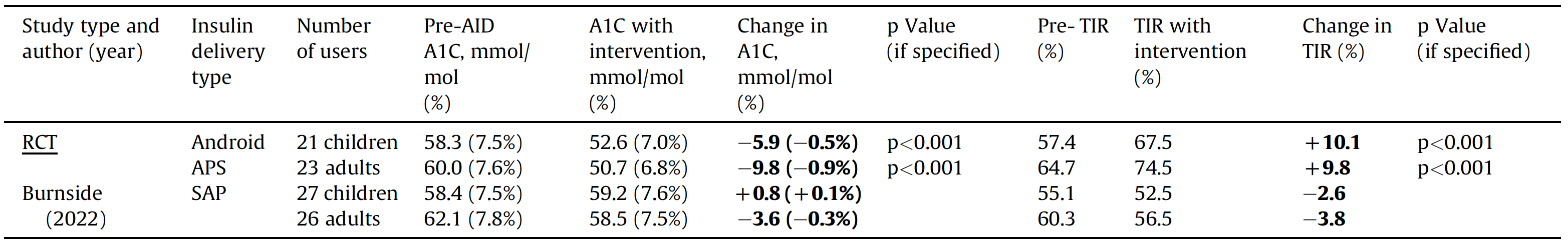
A1C, glycated hemoglobin; AID, automated insulin delivery; DIY, do-it-yourself; SAP, sensor-augmented pump.
Safety of DIY AID
Despite lower mean glucose levels with DIY AID use, this has not been accompanied by an increase in TBR [4,24,25,31–33,35,38,40,44]. In the 6-month RCT, no episodes of severe hypoglycemia or diabetic ketoacidosis occurred in AndroidAPS users [41]. Occurrence of severe hyperglycemia and ketosis (capillary glucose level >17 mmol/L; ketones >1.5 mmol/L and symptomatic) per 100 user-days in the 24-week trial period was 0.10 in the AID group and 0.07 in the control group [42]. Indeed, the lived diabetes experience of DIY AID builders and their immediate need for a safer way of insulin delivery was a strong motivating force contributing to DIY AID development.
Accessibility and costs
Access to insulin pumps and CGM devices varies substantially between Canadian provinces. Some provinces have broad public coverage for pumps, others have broad coverage for CGM, others cover both or neither, while some coverage may be restricted to children and young adults. The prospect of losing coverage when reaching a certain age results in anxiety and financial burden for families. Even with private insurance (e.g. through employers), reimbursement is usually subject to an annual cap and/or co-pays. The resulting out-of-pocket costs may mean that commercial AID systems are not affordable. In contrast, DIY AID can be assembled with out-of-warranty devices that are often less expensive to obtain or already in hand. PWD should be aware that ongoing costs of CGM and infusion set supplies are the same whether using a commercial or DIY system.
The fictional yet realistic cases in Textbox 2 illustrate the complex financial challenges facing PWD in different provinces, even for those with private benefit plans.
Special Populations
Pregnancy
Pregnancy in PWD is associated with an increased rate of both obstetrical and neonatal adverse outcomes. Stringent glycemic targets are imperative, but are often unmet, or can only be achieved with an increased rate of hypoglycemia [45]. The pre-set glycemic targets of currently approved commercial AID systems in Canada are not sufficiently stringent to reach targets recommended for pregnancy. In contrast, customized glucose targets appropriate for pregnancy can be selected by DIY AID users. To date, the literature is limited to small case series and case reports [46–51] that have demonstrated higher percentage TIR with lower percentage TBR compared to previous studies of CGM alone. Safe use of DIY AID at time of birth and postpartum can reduce management burden at a critical time compared to users' pregnancy experience with open-loop insulin pump with CGM [51]. The Expert Working Group’s clinical experience (n=4) also suggests that DIY AID can be used safely and effectively in pregnancy and may yield more time in the target range without severe hypoglycemia, which would be expected to result in fewer adverse pregnancy outcomes.
In-hospital
There have been no published studies on the use of DIY AID while PWD are admitted to hospital. Clinicians should follow local institutional practices regarding self-management in hospitals. There are published protocols that can be used to support safe self-management of diabetes in non-critically ill, psychologically stable PWD [52], and clinicians could apply protocols developed for continuous subcutaneous insulin infusion (CSII) users to individuals who are capable of self-management and using DIY AID.
Pediatrics
The advantages of DIY AID over SAP for glycemia in a 6-month RCT described earlier applied equally to pediatric individuals (aged 7 to 15 years). The DIY AID systems have shown improvements in glycemic management comparable to adult groups [3,10,53]. Additionally, findings reveal improved sleep quality, quality of life, physical health, and less worry about hypoglycemia at night and outside the family home, leading to increased autonomy for the child [53]. Parents and caregivers repeatedly report receiving practical and emotional support from the online community #WeAreNotWaiting [3,53].
As with adult groups, it is vital that pediatric individuals and their parents/caregivers are supported by their diabetes care teams. This should include addressing the need for support in schools and other care settings that is individualized and equitable.
Textbox 2. Three fictional yet realistic cases
Sarah is a 36-year-old mother of 3 living with T1D in Montreal, Quebec. She works as an actuary at a major life insurance company and plays racquetball and squash regularly with her partner to maintain fitness and manage stress. She is too old to benefit from Quebec’s provincial pump coverage, but was pleased when the province listed CGM on the provincial formulary, as that forced her private employer-based insurance to cover a Dexcom G6 system at equivalent or better conditions as the province’s pharmacare plan offers. She pays about $60 out of pocket each month for a Dexcom G6 subscription, with the remaining $240 per month covered by her insurance. Her private insurance covers 80% of durable medical equipment, up to a maximum of $5,000 purchase cost per year. Her partner’s insurance does not include durable medical equipment as a benefit. This means that if Sarah buys a new insulin pump for approximately $8,000, her insurance will cover $4,000 of the cost, and she will have to pay the remaining $4,000. Although she could claim this amount on her federal and provincial taxes to defray the total outlay, the deduction would still leave her with a substantial out-of-pocket expense. Sarah has chosen to build herself a DIY AID system, using her Dexcom G6 system, an old Medtronic pump donated by a friend with T1D, and a linking device she purchased for about $150. Sarah’s total out-of-pocket cost to set up her new system was $150, compared to about $4,000 she would have needed to pay for a commercial AID system. In the 2 years she has been using DIY AID, Sarah has increased her TIR from an average of 72% to an average of 89%, and has brought her A1C from 6.7% to 5.8%.
Ahmed is an 8-year-old boy with T1D whose family lives in Yorkton, Saskatchewan. His school will provide treatments for hypoglycemia in the event of an urgent low, but is unable to provide support to help him administer insulin at lunchtime or at other points during the day. His parents and health-care team believe he is too young to be fully independent in his management. Ahmed’s family was very hopeful about the potential for a Tandem Control-IQ commercial AID system to help Ahmed maintain stable blood glucose levels, feel good at school, and be able to function and learn well. According to Ahmed’s health-care team, he qualifies for provincial coverage for the system. However, without anyone at the school to help him use the system, either his family would need to have a parent come to school every day at lunch, which would mean 1 of his parents would need to leave their job, or they would need to hire an aide to help Ahmed. Each of these options would have a substantial negative financial impact on the family. His parents, therefore, investigated other options, and decided to build a DIY AID system to connect his provincially funded Omnipod and Dexcom G6. The DIY AID system allows Ahmed’s parents to remotely monitor and control his devices through an open-source software platform called Nightscout. They are able to send text messages to school staff in the event of urgent concerns. While the current setup is not always as smooth as they would like and requires substantial parental involvement, the family feels that this system is the most feasible and affordable approach for them to support Ahmed and keep him healthy as he grows towards independence. Since using the DIY AID system, Ahmed’s TIR has increased from 56% to 67% and his A1C has gone from 7.8% to 7.5%.
George is a 68-year-old former nuclear plant operator who retired with his wife from their previous home in Owen Sound, Ontario, to live in Summerside, Prince Edward Island (PEI), near their eldest child and grandchildren. George has lived with T1D for 30 years, having been initially misdiagnosed as having type 2 diabetes. Since his initial diagnosis, George has actively pursued lifestyle changes to manage his diabetes. Once he got the correct diagnosis of T1D and began using intensive insulin therapy, he sought out technology to help him live his best life and be there long-term for his family. While living in Ontario, George was able to start using an insulin pump. His pump and supplies were funded by Ontario’s provincial Assistive Devices Program. Now that he lives in PEI, he is no longer eligible for provincial funding, though he does have some ongoing private coverage for supplies through his workplace retirement benefits. He uses this coverage to partially fund a Libre flash glucose monitoring system. Upon learning that 1 of his old Medtronic pumps was compatible with DIY AID systems, he built himself such a system, using his old Medtronic pump, a Libre flash glucose monitor, and 2 aftermarket devices he bought for $100-$150 each, 1 to turn the Libre from a flash to a continuous monitor, and 1 to allow his smartphone to send commands to his pump. This financial outlay was affordable to George and his wife, whereas a commercial system would be well beyond their retirement budget. George’s blood glucose metrics have not changed markedly with his new system. As he has always aimed to do, he maintains his A1C between 6.5% and 7.0%. His TIR is typically around 80%. However, he has noticed a marked improvement in his sleep, with a much-improved ability to sleep through the night without needing to wake up to attend to a blood glucose excursion. This has also helped his wife sleep better, as she used to wake to get him juice when he was low, an event that happens much less often now.
Ethical and Medico-legal Considerations
Medical ethics
HCPs have a professional obligation to serve the public good by making the health and well-being of the individuals in their practice their first consideration. A second key aspect of the HCP–PWD relationship is that of respect for individual autonomy. These ethical principles require PWD to be provided with information about available therapeutic tools (treatment options) that can reasonably be expected to be beneficial for them, including those that might not be recommended (for instance because they are less effective or have more side effects, or because of costs and coverage).
PWD can only make fully informed decisions when they have been provided with information about all treatment options that can reasonably be expected to be beneficial to them. Withholding information undermines autonomy, a key value supported in the Canadian Medical Association Code of Ethics, as well as the provincial colleges of most regulated health professionals [54–56]. All members of the diabetes care team play a role in discussions around therapy choices, and will often be the first point of contact for troubleshooting. The same ethical principles apply to all HCPs in the unique scenario of supporting PWD who choose to use DIY therapy.
Medico-legal considerations
Autonomy and equitable access to health care are overriding legal rights of individuals in Canadian law and jurisprudence. There is a compelling legal argument that HCPs who support PWD who are using DIY AID are acting within the law in Canada. Conversely, refusal to provide support without making an effective referral to another HCP who can and will support the individual—and is accessible to the individual—may, in fact, be in violation of the law. Refusing to provide medical support for PWD who choose to use DIY AID is a form of gatekeeping that leads to the effective denial of equitable access to health care, creating a risk of significant bodily harm [57–59].
Another concern for HCPs is potential liability for negligence if they are providing medical support to PWD using DIY AID. A review of the legal test to determine negligence discloses that there may, in fact, be a greater liability from a refusal to provide medical support to people using DIY AID than to supporting use of DIY AID. A claim of negligence requires the plaintiff to prove on balance of probabilities that (1) the clinician had a duty of care, (2) the clinician breached the standard of care, (3) the plaintiff sustained damage, and (4) the plaintiff’s damage was directly caused by the defendant’s breach of the standard of care [58]. All 4 arms of the test must be met in order for a clinician to be found negligent, which is more likely to be the case if the PWD was denied access to care due to their own choice to use DIY AID.
For HCPs who prefer not to provide ongoing medical support for PWD who choose to use DIY AID, it is advisable to provide a referral to a clinician with experience. The use of virtual care should be encouraged to facilitate PWD from rural and remote communities to access clinicians with experience using commercial and DIY AID systems. Overall, a review of the legal literature, liability seems to be a greater risk if clinicians refuse to discuss, support, or refer on for DIY AID rather than the often cited concern that supporting individuals using these technologies opens clinicians up to liability claims.
The Canadian Agency for Drugs and Technology in Health (CADTH) defines ”off-label,” or expanded use, as any use beyond that which Health Canada has reviewed and authorized to be marketed in Canada. CADTH deems off-label prescribing to be safe, provided there is strong scientific evidence to support it, with a balance of risk and benefit considered [60]. While the CGM and insulin pumps used in DIY AID systems have Health Canada approval, the computer code that makes insulin adjustments does not. However, as discussed above, there is ample evidence of effectiveness and all discussions should review the benefits and risks in the context of the PWD’s goals. The Canadian Medical Protective Agency has recommendations for physicians when recommending the use of off-label drugs and devices [61] (Table 3).
To be clear, we recommend that PWD take responsibility for the technical hardware and computer coding. This should not be the responsibility of the clinician. If a clinic or third party does the initial build, the PWD may lack the necessary troubleshooting skills. HCPs should inform PWD that there is no helpline to call when there are technical failures with DIY AID and when there are questions about system features the online community is where answers will be found. Another concern raised is about the use of out-of-warranty devices in some DIY AID systems, but this is the reality assumed by many pump users whose warranty may expire before they are eligible for a new pump, depending on insurance funding cycles. As with all individuals using an insulin pump, clinicians should advise DIY AID users to have a backup plan for insulin delivery in the case of system failure. While we recognize that not all clinicians will be comfortable with AID, we feel that if AID is going to be offered then DIY AID should be mentioned alongside commercial options. If clinicians are comfortable supporting individuals on commercial systems, they should be comfortable supporting individuals on DIY systems as well. In the future, we hope to see the various DIY systems receive approval from regulatory bodies to open more options for interoperability and allow for more widespread use, as was done by Tidepool Loop, which received FDA clearance in January 2023 but has yet to announce its pump and CGM partners as of the printing of this position statement [62].
Supporting DIY AID Use in Practice
While PWD may independently build their DIY AID systems, the diabetes care team remains essential for core diabetes self-management education and support for DIY AID use. Core skills in diabetes management, insulin pump therapy use, and CGM use provide the foundation for success with DIY AID systems. Clinicians should prioritize learning the key system characteristics to facilitate supporting PWD in optimizing settings to safely and effectively help them meet glycemic and personal goals. See the corresponding user's guide (page 389) for guidance on how the systems work, optimizing settings, key educational points, and available resources.
Summary and Key Messages
The evidence supports that DIY AID can provide significant improvements in A1C, TIR, and quality of life. The evidence also suggests that a wide range of PWD can benefit from AID regardless of their education or comfort with technology [14]. In the spirit of shared decision-making and facilitating self-management, and in keeping with the ethical and legal principles of duty of care and autonomy, HCPs are obligated to discuss all available options with PWD, including making them aware of DIY AID. This does not mean that HCPs need to become experts in the setup and building of a DIY system, but rather that HCPs direct PWD to the online diabetes community to learn more about the DIY systems and support them at follow-up appointments to adjust and optimize settings. Reviewing the glucose and insulin data should still be a part of all routine follow-up. HCPs and PWD should agree on how to review the data and make joint decisions on how to further improve TIR, decrease glycemic variability and meet the PWD goals. A companion user's guide has been published for HCPs who wish to learn how these systems work and develop an approach to helping PWD meet their personalized diabetes goals.
Effectiveness of DIY AID
- DIY AID can be an effective approach to support individuals with T1D to increase TIR and reduce A1C without increasing hypoglycemia, all while reducing the burden of diabetes self-management and improving quality of life.
- The benefits revealed by qualitative studies and lived experience should be carefully considered alongside glycemic benefits when discussing the risks and benefits of DIY AID. A net assessment of total risk should include the goals and concerns of the PWD and not overlook the risks inherent in all methods of insulin delivery.
- No comparative studies have evaluated commercial versus DIY AID systems, such that no assumptions can be made about the superiority in either direction. Individual needs and preferences should direct choices.
Role of HCPs
- HCPs have an obligation to discuss all available treatment options that have evidence of benefit with individuals living with T1D. DIY AID should be discussed alongside commercially approved options.
- DIY AID may be more accessible to PWD in Canada than commercial systems due to costs, variation in coverage, and slow approval of commercial systems. Out-of-pocket costs should be considered even for those with private insurance.
- HCPs should be aware of their biases and the potential for inappropriate gatekeeping (e.g. HCPs making assumptions that an individual or family is not equipped to manage DIY AID). Traditional “prerequisites” for pump use may not be relevant in DIY AID (e.g. precise carb counting, frequent finger sticks).
- HCPs should make every effort to support PWD who choose to use DIY AID and seek to maintain the therapeutic alliance. HCPs should facilitate effective referrals if an individual’s needs exceed the expertise of the HCP.
- It is recommended that PWD should build and install their own AID software. HCPs may support PWD in building their own systems, for example, by providing clinic space for peer-led mentorship programs (e.g. “build parties”) and encouraging engagement with peer-to-peer technical support networks.
- HCPs should continue to provide ongoing support and education on core diabetes self-management skills, and safe use of insulin pump therapy and CGM.
- HCPs should assist with optimizing pump settings and coaching on the behaviours linked to improving glycemic outcomes and meeting personal goals.
Role of the Community
- Where possible, HCPs should connect interested PWD to trusted online, social media, and in-person resources. Peer support has been an effective tool both for people seeking to start using DIY AID and for providing help and support to existing users.
- Additional adjustable parameters in newer branches of DIY algorithms may include terminology that does not build on existing CSII settings language, making it difficult for HCPs to support individuals in optimizing settings. An effort by the community of developers to consult with the diabetes education community would advance translation of DIY AID into clinical practice.
External Reviewers
Thank you to our external reviewers for their insightful feedback and the lending of their time and expertise:
Lois Donovan MD, FRCPC
Bruce A. Perkins MD, FRCPC
Author Disclosures
I.J.H. has received speakers and advisory board fees from Dexcom Canada, Abbott Diabetes Canada, Novo Nordisk, and Sanofi unrelated to the current work; H.O.W. is funded by a Canada Research Chair in Human-Centred Digital Health, has lived with type 1 diabetes since 1983, and has used a DIY AID since 2019; A.C. has received speaker fees from Dexcom Canada, Abbott Diabetes Canada, consulting fees from Dexcom Canada, and insulin pump training fees from Insulet, Medtronic, and Tandem, she lives with type 1 diabetes, and has personal experience using both DIY AID and commercially available AID systems (Medtronic and Tandem); P.S. is supported by the Charles A. Allard Chair in Diabetes Research and the Alberta Academic Medicine and Health Services Plan and has received personal fees for consulting for Vertex, Novo Nordisk, and Viatris; K.F. is a member of the Nightscout Foundation board of directors; U.S., A.E.M., and L.C. have no conflicts.
∗Throughout this statement, the term PWD will be used to refer to people living with T1D and their caregivers.
References
- Weisman A, Perkins BA. Attainment of glycemic targets among adults with diabetes in Canada: A cross-sectional national diabetes repository study. Diabetes 2021;70(Suppl. 1):1062-P.
- American Diabetes Association Professional Practice Committee. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022;45(Suppl. 1): S97-112.
- Braune K, Lal RA, Petruzelkova L, Scheiner G, Winterdijk P, Schmidt S, Raimond L, Hood KK, Riddell MC, Skinner TC, Raile K, Hussain S; OPEN International Healthcare Professional Network and OPEN Legal Advisory Group. Open-source automated insulin delivery: international consensus statement and practical guidance for health-care professionals. Lancet Diabetes Endocrinol. 2022 Jan;10(1):58-74. doi: 10.1016/S2213-8587(21)00267-9. Epub 2021 Nov 13. Erratum in: Lancet Diabetes Endocrinol. 2022 Jan;10(1):e1. PMID: 34785000; PMCID: PMC8720075.
- Morrison AE, Chong K, Lai V, Senior PA, Lam A. Improved glycemia and quality of life among loop users---a retrospective analysis of real-world data from a single centre. Diabetes Technol Ther 2022;24:A47.
- Morrison AE, Farnsworth K, Witteman HO, Lam A, Senior PA. Canadian healthcare providers' attitudes towards automated insulin delivery systems. medRxiv 2022.
- Diabetes Australia. People with type 1 diabetes and do-it-yourself (DIY) technology solutions. https://www.diabetesaustralia.com.au/wp-content/uploads/do-it-yourself-solutions-type-1-diabetes.pdf. Accessed December 1, 2022.
- Morrison AE, et al. Do-it-yourself and commercial automated insulin delivery systems in type 1 diabetes: An uncertain area for Canadian healthcare providers. Can J Diabetes 2022.
- Diabetes Canada. Diabetes Canada Clinical Practice Guidelines: Processes manual. 2021.
- Morrison AE, et al. A scoping review of do-it-yourself automated insulin delivery system (DIY AID) use in people with type 1 diabetes. PLoS One 2022;17:e0271096.
- Suttiratana SC, Wong JJ, Lanning MS, Dunlap A, Hanes SJ, Hood KK, Lal RA, Naranjo D. Qualitative Study of User Experiences with Loop, an Open-Source Automated Insulin Delivery System. Diabetes Technol Ther. 2022 Jun;24(6):416-423. doi: 10.1089/dia.2021.0485. Epub 2022 May 12. PMID: 35099278; PMCID: PMC9208860.
- Crocket H, Lewis DM, Burnside M, Faherty A, Wheeler B, Frewen C, Lever C, Jefferies C, Williman J, Sanders O, Wilson R, Paul R, Price S, Jones S, de Bock M. Learning challenges of healthcare professionals supporting open-source automated insulin delivery. Diabet Med. 2022 May;39(5):e14750. doi: 10.1111/dme.14750. Epub 2021 Dec 7. PMID: 34826158.
- Shepard JA, Breton M, Nimri R, Roberts JTF, Street T, Klonoff D, Barnard-Kelly K. User and Healthcare Professional Perspectives on Do-It-Yourself Artificial Pancreas Systems: A Need for Guidelines. J Diabetes Sci Technol. 2022 Jan;16(1):224-227. doi: 10.1177/1932296820957728. Epub 2020 Oct 1. PMID: 33000636; PMCID: PMC8875047.
- Dinneen SF, McMorrow L. Re-framing type 1 diabetes care through open-source automated insulin delivery: 'The (expert) patient will see you now, doctor.' Diabet Med 2022;39:e14839.
- Lawton J, Kimbell B, Rankin D, Ashcroft NL, Varghese L, Allen JM, Boughton CK, Campbell F, Randell T, Besser REJ, Trevelyan N, Hovorka R; CLOuD Consortium. Health professionals' views about who would benefit from using a closed-loop system: a qualitative study. Diabet Med. 2020 Jun;37(6):1030-1037. doi: 10.1111/dme.14252. Epub 2020 Feb 14. PMID: 31989684.
- Looped Facebook Group. http://www.facebook.com/groups/theloopedgroup. Accessed May 26, 2022.
- Crocket H. Peer mentoring in the do-it-yourself artificial pancreas system community. J Diabetes Sci Technol 2020;14:1022-1027.
- Farnsworth K, S.M , Drescher O, Racine C, Senior P, Witteman H. Who is using do-it-yourself artificial pancreas systems and why. Advanced Technologies and Treatment for Diabetes Conference. The Official Journal of ATTD Advanced Technologies & Treatments for Diabetes CONFERENCE 27-30 April 2022 I BARCELONA & ONLINE. Diabetes Technology & Therapeutics.Apr 2022.A-1-A-237.http://doi.org/10.1089/dia.2022.2525.abstracts
- Schipp J, Skinner TC, Holloway E, Scibilia R, Langstrup H, Speight J, Hendrieckx C. 'We're all on the same team'. Perspectives on the future of artificial pancreas systems by adults in Australia with type 1 diabetes using open-source technologies: A qualitative study. Diabet Med. 2022 May;39(5):e14708. doi: 10.1111/dme.14708. Epub 2021 Oct 7. PMID: 34599617.
- Braune K, O’Donnell SH, Cleal B, Lewis DM, Tappe A, Hauck B, et al. 117-LB: DIWHY: factors influencing motivation, barriers, and duration of DIY artificial pancreas system use among real-world users. Diabetes 2019;68(Suppl. 1):XXX.
- Huhndt A, et al. Barriers to uptake of open-source automated insulin delivery systems: Analysis of socioeconomic factors and perceived challenges of caregivers of children and adolescents with type 1 diabetes from the OPEN survey. Front Clin Diabetes Healthc 2022. 3.
- Wong JJ, Suttiratana SC, Lal RA, Lum JW, Lanning MS, Dunlap A, Arbiter B, Hanes SJ, Bailey RJ, Hood KK, Naranjo D. Discontinued Use of the Loop Insulin Dosing System: A Mixed-Methods Investigation. Diabetes Technol Ther. 2022 Apr;24(4):241-248. doi: 10.1089/dia.2021.0362. PMID: 34780283; PMCID: PMC9057870.
- https://openaps.org/outcomes/. Accessed June 1, 2023.
- Lewis D, S. Leibrand, and A.P.S.C. Open, real-world use of open source artificial pancreas systems. J Diabetes Sci Technol 2016;XX:1411.
- Lewis DM, Swain RS, Donner TW. Improvements in A1C and time-in-range in DIY closed-loop (OpenAPS) users. Diabetes 2018;67(Suppl. 1):XXX.
- Provenzano V, Guastamacchia E, Brancato D, Cappiello G, Maioli A, Mancini R, et al. Closing the loop with OpenAPS in people with type 1 diabetes---experience from Italy. Diabetes 2018;67(Suppl. 1):XXX.
- Braune K, O'Donnell S, Cleal B, Lewis D, Tappe A, Willaing I, Hauck B, Raile K. Real-World Use of Do-It-Yourself Artificial Pancreas Systems in Children and Adolescents With Type 1 Diabetes: Online Survey and Analysis of Self-Reported Clinical Outcomes. JMIR Mhealth Uhealth. 2019 Jul 30;7(7):e14087. doi: 10.2196/14087. PMID: 31364599; PMCID: PMC6691673.
- Melmer A, Zuger T, Lewis DM, Leibrand S, Stettler C, Laimer M. Glycaemic control in individuals with type 1 diabetes using an open source artificial pancreas system (OpenAPS). Diabetes Obes Metab. 2019 Oct;21(10):2333-2337. doi: 10.1111/dom.13810. Epub 2019 Jul 5. PMID: 31183929.
- Braune K, Gajewska KA, Thieffry A, Lewis DM, Froment T, O'Donnell S, Speight J, Hendrieckx C, Schipp J, Skinner T, Langstrup H, Tappe A, Raile K, Cleal B. Why #WeAreNotWaiting-Motivations and Self-Reported Outcomes Among Users of Open-source Automated Insulin Delivery Systems: Multinational Survey. J Med Internet Res. 2021 Jun 7;23(6):e25409. doi: 10.2196/25409. PMID: 34096874; PMCID: PMC8218212.
- Street TJ. Review of self-reported data from UK do-it-yourself artificial pancreas system (DIYAPS) users to determine whether demographic of population affects use or outcomes. Diabetes Ther 2021;12:1839-1848.
- Petruzelkova L, Soupal J, Plasova V, Jiranova P, Neuman V, Plachy L, Pruhova S, Sumnik Z, Obermannova B. Excellent Glycemic Control Maintained by Open-Source Hybrid Closed-Loop AndroidAPS During and After Sustained Physical Activity. Diabetes Technol Ther. 2018 Nov;20(11):744-750. doi: 10.1089/dia.2018.0214. Epub 2018 Oct 4. PMID: 30285476.
- Choi SB, Hong ES, Noh YH. Open artificial pancreas system reduced hypoglycemia and improved glycemic control in patients with type 1 diabetes. Diabetes 2018;67(Suppl. 1):XXX.
- Wu Z, Luo S, Zheng X, Bi Y, Xu W, Yan J, Yang D, Weng J. Use of a do-it-yourself artificial pancreas system is associated with better glucose management and higher quality of life among adults with type 1 diabetes. Ther Adv Endocrinol Metab. 2020 Aug 25;11:2042018820950146. doi: 10.1177/2042018820950146. PMID: 32922721; PMCID: PMC7453453.
- Lum JW, Bailey RJ, Barnes-Lomen V, Naranjo D, Hood KK, Lal RA, Arbiter B, Brown AS, DeSalvo DJ, Pettus J, Calhoun P, Beck RW. A Real-World Prospective Study of the Safety and Effectiveness of the Loop Open Source Automated Insulin Delivery System. Diabetes Technol Ther. 2021 May;23(5):367-375. doi: 10.1089/dia.2020.0535. Epub 2021 Apr 12. PMID: 33226840; PMCID: PMC8080906.
- Petruzelkova L, Jiranova P, Soupal J, Kozak M, Plachy L, Neuman V, Pruhova S, Obermannova B, Kolouskova S, Sumnik Z. Pre-school and school-aged children benefit from the switch from a sensor-augmented pump to an AndroidAPS hybrid closed loop: A retrospective analysis. Pediatr Diabetes. 2021 Jun;22(4):594-604. doi: 10.1111/pedi.13190. Epub 2021 Feb 25. PMID: 33576551.
- Gawrecki A, Zozulinska-Ziolkiewicz D, Michalak MA, Adamska A, Michalak M, Frackowiak U, Flotynska J, Pietrzak M, Czapla S, Gehr B, Araszkiewicz A. Safety and glycemic outcomes of do-it-yourself AndroidAPS hybrid closed-loop system in adults with type 1 diabetes. PLoS One. 2021 Apr 5;16(4):e0248965. doi: 10.1371/journal.pone.0248965. PMID: 33819289; PMCID: PMC8021167.
- Jeyaventhan R, Gallen G, Choudhary P, Hussain S. A real-world study of user characteristics, safety and efficacy of open-source closed-loop systems and Medtronic 670G. Diabetes Obes Metab. 2021 Aug;23(8):1989-1994. doi: 10.1111/dom.14439. Epub 2021 Jun 9. PMID: 33999488.
- Crabtree TS, Hussain S, Mendis B, Gazis T, Herring R, Idris I, Ryder R, Wilmot EG. Glycaemic and safety outcomes associated with do-it-yourself artificial pancreas systems (DIYAPS): Initial insights from the Association of British Clinical Diabetologist's (ABCD) DIYAPS Audit Programme. Diabetes Technol Ther 2022;24:A57.
- Amuedo S, Antequera M, Azriel S. 800-P: Real-world use of do-it-yourself artificial pancreas systems in adults with type 1 diabetes. Diabetes 2022;71(Suppl. 1):XXX.
- Mueller-Korbsch M, Fruehwald L, Kietaibl AT. 767-P: Changes in ambulatory glucose profile (AGP) in patients with type 1 diabetes mellitus after switching from sensor-augmented insulin pump therapy to a do-it-yourself artificial pancreas system: A retrospective data analysis of real-world data. Diabetes 2022;71(Suppl. 1):XXX.
- Patel R, C.T.S.J. and et al., Glycemic outcomes of do-it-yourself artificial pancreas systems (DIY APS) verses Freestyle Libre (FSL) with concomitant insulin pump therapy (CSII): A cross-sectional comparison from the Royal Derby Hospital cohort, in Diabetes UK. 2021.
- Burnside MJ, Lewis DM, Crocket H, Meier R, Williman J, Sanders OJ, et al. 286-OR: The CREATE trial: Randomized clinical trial comparing open-source automated insulin delivery with sensor augmented pump therapy in type 1 diabetes. Diabetes 2022;71(Suppl. 1):XXX.
- Burnside MJ, et al. Open-source automated insulin delivery in type 1 diabetes. N Engl J Med 2022;387:869-881.
- Hood KK, Wong JJ, Hanes S, Bailey R, Calhoun P, Beck R, et al. 1001-P: Do-it-yourself (DIY) loop is associated with psychosocial benefits. Diabetes 2020;69(Suppl. 1):XXX.
- Jiranova P, Soupal J, Plachy L, Neumann V, Sumnik Z, Kozak M, et al. An AndroidAPS Hybrid Closed Loop system in a home setting is safe and leads to better metabolic control. Diabetes Technol Ther 2019;21:A84-A85.
- Murphy HR. Continuous glucose monitoring targets in type 1 diabetes pregnancy: Every 5% time in range matters. Diabetologia 2019;62:1123-1128.
- Schutz AK, et al. Management of type 1 diabetes mellitus using open-source automated insulin delivery during pregnancy: A case series. Diabetes Technol Ther 2022;24:227-230.
- Schutz-Fuhrmann I, et al. Two subsequent pregnancies in a woman with type 1 diabetes: Artificial pancreas was a gamechanger. J Diabetes Sci Technol 2020;XX:972-973.
- Bukhari K, Malek R. Open-source automated insulin delivery systems for the management of type 1 diabetes during pregnancy. BMJ Case Reports CP 2021;14:e243522.
- Waikar AR, Arora T, Haynes M, Tamborlane WV, Nally LM. Case Report: Managing Pregnancy With Type 1 Diabetes Using a Do-It-Yourself Artificial Pancreas System. Clin Diabetes. 2021 Oct;39(4):441-444. doi: 10.2337/cd20-0128. PMID: 34866788; PMCID: PMC8603326.
- Treiber G, N.E.H.E. and J. Mader. Challenges with DIY loop in pregnancy with type one diabetes [abstract]. In: Advanced Technologies & Treatments for Diabetes (ATTD); June 2-5, 2021 (virtual).
- Lemieux P, Yamamoto JM, Donovan LE. Do-it-yourself artificial pancreas system use in pregnant women with type 1 diabetes in a real-world setting: 2 Case reports. Can J Diabetes 2021;45:804-8. e2.
- Halperin I, Malcolm J, Moore S, Houlden RL; Canadian Standards for Perioperative/Periprocedure Glycemic Management Expert Consensus Panel. Suggested Canadian Standards for Perioperative/Periprocedure Glycemic Management in Patients With Type 1 and Type 2 Diabetes. Can J Diabetes. 2022 Feb;46(1):99-107.e5. doi: 10.1016/j.jcjd.2021.04.009. Epub 2021 May 3. PMID: 34210609.
- Braune K, Krug N, Knoll C, Ballhausen H, Thieffry A, Chen Y, et al. Emotional and physical health impact in children and adolescents and their caregivers using open-source automated insulin delivery: Qualitative analysis of lived experiences. J Med Internet Res 2022;24:e37120.
- CMA Code of Ethics and Professionalism. 2018.
- Canadian Nurses Association. Code of Ethics for Registered Nurses 2017.
- BC College of Dietitians. Code of Ethics Principles and Guidelines. Revised 2012.
- Christian Medical and Dental Society of Canada v. College of Physicians and Surgeons of Ontario, 2019 ONCA 393 (CanLII), <https://canlii.ca/t/j08wq>, retrieved on 2023-07-20.
- Mustapha v. Culligan of Canada Ltd., 2008 S.C.C. 27 (2008).
- R. v. Morgentaler, 1988 CanLII 90 (SCC), [1988] 1 SCR 30, <https://canlii.ca/t/1ftjt>, retrieved on 2023-07-20; at 56-60, per Dickson CJ and Lamer J, concurring; Dunn Affidavit, p 4149, para 24(c).
- CADTH Tool: Off-label drug use. December 27, 2021. https://www.cadth.ca/sites/default/files/pdf/off_label_use_of_drugs_pro_e.pdf.
- Association, T.C.M.P. Using medications safely: Strategies for managing risks. Accessed Jan 21 2023]; Available from: https://www.cmpa-acpm.ca/en/education-events/good-practices/the-healthcare-system/medication-safety?panel=medication-reconciliation&utm_source=cis-en&utm_medium=email&utm_campaign=connect-1.
- Tide Pool. https://www.tidepool.org/tidepool-loop. Accessed January 29, 2023.
*The Canadian Diabetes Association is the registered owner of the name Diabetes Canada. All content on guidelines.diabetes.ca, CPG Apps and in our online store remains exactly the same. For questions, contact communications@diabetes.ca.


