Chapter Headings
- Introduction
- Ethnocultural Diversity
- Approach to Nutrition Therapy
- Energy
- Macronutrients
- Intensive Lifestyle Intervention
- Dietary Patterns
- Diets Emphasizing Specific Foods
- Special Considerations for People with Type 1 Diabetes and Type 2 Diabetes on Insulin
- Other Considerations
- Other Relevant Guidelines
- Author Disclosures
Key Messages
- People with diabetes should receive nutrition counselling by a registered dietitian.
- Nutrition therapy can reduce glycated hemoglobin (A1C) by 1.0% to 2.0% and, when used with other components of diabetes care, can further improve clinical and metabolic outcomes.
- Reduced caloric intake to achieve and maintain a healthier body weight should be a treatment goal for people with diabetes with overweight or obesity.
- The macronutrient distribution is flexible within recommended ranges and will depend on individual treatment goals and preferences.
- Replacing high-glycemic-index carbohydrates with low-glycemic-index carbohydrates in mixed meals has a clinically significant benefit for glycemic control in people with type 1 and type 2 diabetes.
- Consistency in spacing and intake of carbohydrate intake and in spacing and regularity in meal consumption may help control blood glucose and weight.
- Intensive healthy behaviour interventions in people with type 2 diabetes can produce improvements in weight management, fitness, glycemic control and cardiovascular risk factors.
- A variety of dietary patterns and specific foods have been shown to be of benefit in people with type 1 and type 2 diabetes.
- People with diabetes should be encouraged to choose the dietary pattern that best aligns with their values, preferences and treatment goals, allowing them to achieve the greatest adherence over the long term.
Key Messages for People with Diabetes
- It is natural to have questions about what food to eat. A registered dietitian can help you develop a personalized meal plan that considers your culture and nutritional preferences to help you achieve your blood glucose and weight management goals.
- Food is key in the management of diabetes and reducing the risk of heart attack and stroke.
- Try to prepare more of your meals at home and use fresh unprocessed ingredients.
- Try to prepare meals and eat together as a family. This is a good way to model healthy food behaviours to children and teenagers, which could help reduce their risk of becoming overweight or developing diabetes.
- With prediabetes and recently diagnosed type 2 diabetes, weight loss is the most important and effective dietary strategy if you have overweight or obesity. A weight loss of 5% to 10% of your body weight may help normalize blood glucose levels.
- There are many strategies that can help with weight loss. The best strategy is one that you are able to maintain long term.
- Adoption of diabetes-friendly eating habits can help manage your blood glucose levels as well as reduce your risk for developing heart and blood vessel disease for those with either type 1 or type 2 diabetes.
- Select whole and less refined foods instead of processed foods, such as sugar-sweetened beverages, fast foods and refined grain products.
-
Pay attention to both carbohydrate quality and quantity.
- Include low-glycemic-index foods, such as legumes, whole grains, and fruit and vegetables. These foods can help control blood glucose and cholesterol levels.
- Consider learning how to count carbohydrates as the quantity of carbohydrate eaten at one time is usually important in managing diabetes.
- Select unsaturated oils and nuts as the preferred dietary fats.
- Choose lean animal proteins. Select more vegetable protein.
- The style of eating that works well for diabetes may be described as a Mediterranean style diet, Nordic style diet, DASH diet or vegetarian style diet. All of these diets are rich in protective foods and have been shown to help manage diabetes and cardiovascular disease. They all contain the key elements of a diabetes-friendly diet.
Introduction
Nutrition therapy and counselling are an integral part of the treatment and self-management of diabetes. The goals of nutrition therapy are to maintain or improve quality of life and nutritional and physiological health; and to prevent and treat acute- and long-term complications of diabetes, associated comorbid conditions and concomitant disorders. It is well documented that nutrition therapy can improve glycemic control (1) by reducing glycated hemoglobin (A1C) by 1.0% to 2.0% (2–5) and, when used with other components of diabetes care, can further improve clinical and metabolic outcomes (3,4,6,7), resulting in reduced hospitalization rates (8).
Ethnocultural Diversity
Canada is a country rich in ethnocultural diversity. More than 200 ethnic origins were reported in Canada in the 2011 census. The most common ethnic origins with populations in excess of 1 million from highest to lowest include Canadian, English, French, Scottish, Irish, German, Italian, Chinese, Aboriginal, Ukrainian, East Indian, Dutch and Polish. The largest visible minorities include South Asians, Chinese and Blacks, followed by Filipinos, Latin Americans, Arabs, Southeast Asians, West Asians, Koreans and Japanese (9). These different ethnocultural groups have distinct and shared foods, food preparation techniques, dining habits, dietary patterns, and lifestyles that directly impact the delivery of nutrition therapy. A “transcultural” approach to nutrition therapy that takes into account these issues has been proposed and has the goal of providing culturally congruent nutrition counselling (10).
Approach to Nutrition Therapy
Nutrition therapy should be individualized, regularly evaluated, reinforced in an intensive manner (11,12), and should incorporate self-management education (13). A registered dietitian (RD) should be involved in the delivery of care wherever possible. Counselling provided by an RD with expertise in diabetes management (14,15), delivered in either a small group and/or an individual setting (16–18), has demonstrated benefits for those with, or at risk for, diabetes. Frequent follow up (i.e. every 3 months) with an RD has also been associated with better dietary adherence in people with type 2 diabetes (7). Individual counselling may be preferable for people of lower socioeconomic status (8), while group education has been shown to be more effective than individual counselling when it incorporates principles of adult education (19). Additionally, in people with type 2 diabetes, culturally sensitive peer education has been shown to improve A1C, nutrition knowledge and diabetes self-management (20), and web-based care management has been shown to improve glycemic control (21). Diabetes education programs serving vulnerable populations should evaluate the presence of barriers to healthy eating (e.g. cost of healthy food, stress-related overeating) (22) and work toward solutions to facilitate behaviour change.
The starting point of nutrition therapy is to follow the healthy diet recommended for the general population based on Eating Well With Canada's Food Guide (22). As the Food Guide is in the process of being updated, specific recommendations are subject to change based on the evidence review and public consultation by Health Canada (https://www.foodguideconsultation.ca/professionals-and-organizations). Current dietary advice is to consume a variety of foods from the 4 food groups (vegetables and fruits; grain products; milk and alternatives; meat and alternatives), with an emphasis on foods that are low in energy density and high in volume to optimize satiety and discourage overconsumption. Following this advice may help a person attain and maintain a healthy body weight while ensuring an adequate intake of carbohydrate (CHO), fibre, fat, protein, vitamins and minerals.
There is evidence to support a number of other macronutrient-, food- and dietary pattern-based approaches. As evidence is limited for the rigid adherence to any single dietary approach (23,24), nutrition therapy and meal planning should be individualized to accommodate the individual's values and preferences, which take into account age, culture, type and duration of diabetes, concurrent medical therapies, nutritional requirements, lifestyle, economic status (25), activity level, readiness to change, abilities, food intolerances, concurrent medical therapies and treatment goals. This individualized approach harmonizes with that of other clinical practice guidelines for diabetes and for dyslipidemia (10,26).
Figures 1 and 2
Energy
Because an estimated 80% to 90% of people with type 2 diabetes have overweight or obesity, strategies that include energy restriction to achieve weight loss are a primary consideration (27). A modest weight loss of 5% to 10% of initial body weight can substantially improve insulin sensitivity, glycemic control, hypertension and dyslipidemia in people with type 2 diabetes and those at risk for type 2 diabetes (28–30). Total calories should reflect the weight management goals for people with diabetes and overweight or obesity (i.e. to prevent further weight gain, to attain and maintain a healthy or lower body weight for the long term or to prevent weight regain).
| Table 1 Properties of dietary interventions* |
|---|
A1C, glycated hemoglobin; apo B, apolipoprotein B; BMI, body mass index; BP, blood pressure; CHD, coronary heart disease; CHO, carbohydrate; CRP, C reactive protein; CV, cardiovascular; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; FPG, fasting plasma glucose; GI, gastrointestinal; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MUFA, monounsaturated fatty acid; SSBs, sugar-sweetened beverages; TC, total cholesterol; TG, triglycerides. |
 |
Figure 2
Stage-targeted nutrition and other healthy behaviour strategies for people with type 2 diabetes.
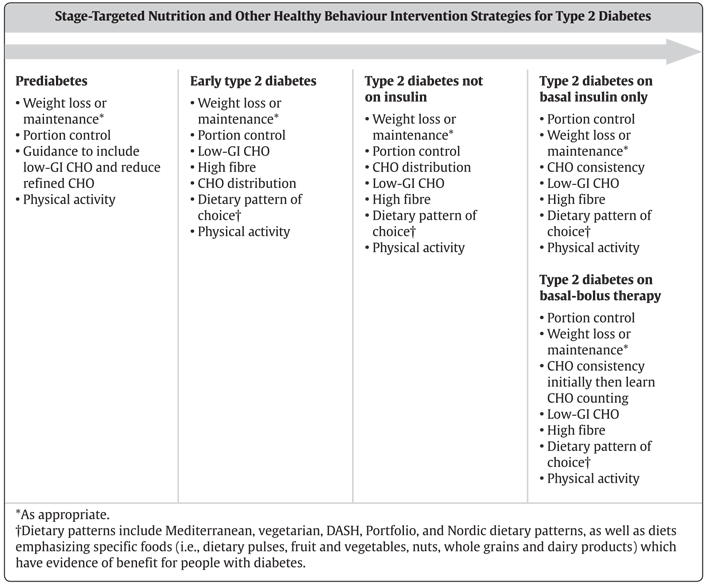
CHO, carbohydrate; GI, glycemic index; NPH, neutral protamine Hagedorn.
Macronutrients
The ideal macronutrient distribution for the management of diabetes may vary, depending on the quality of the various macronutrients, the goals of the dietary treatment regimen and the individual's values and preferences.
Carbohydrate
CHO broadly include available CHO from starches and sugars and unavailable CHO from fibre. The dietary reference intakes (DRIs) specify a recommended dietary allowance (RDA) for available CHO of no less than 130 g/day for adult women and men >18 years of age, to provide glucose to the brain (31). The DRIs also recommended that the percentage of total daily energy from CHO should be ≥45% to prevent high intake of saturated fatty acids as it has been associated with reduced risk of chronic disease for adults (31). If CHO is derived from low glycemic index (GI) and high-fibre foods, it may contribute up to 60% of total energy, with improvements in glycemic and lipid control in adults with type 2 diabetes (32).
Systematic reviews and meta-analyses of controlled trials of CHO-restricted diets (mean CHO of 4% to 45% of total energy per day) for people with type 2 diabetes have not shown consistent improvements in A1C compared to control diets (33–35). Similarly, inconsistent improvements in lipids and blood pressure (BP) have been reported when comparing low-CHO to higher-CHO diets (33–35). As for weight loss, low-CHO diets for people with type 2 diabetes have not shown significant advantages for weight loss over the short term (33,34). There also do not appear to be any longer-term advantages. Although a network systematic review and meta-analysis of randomized controlled trials of popular weight loss diets showed that low-CHO diets (defined as ≤40% energy from CHO) resulted in greater weight loss compared with high-CHO, low-fat diets (defined as ≥60% energy from CHO) at 6 months, there was no difference at 12 months in individuals with overweight or obesity with a range of metabolic phenotypes, including type 2 diabetes (36). Of note, very-low-CHO diets have ketogenic effects that may be a concern for those at risk of diabetic ketoacidosis taking insulin or SGLT2 inhibitors (37) (see Pharmacologic Glycemic Management of Type 2 Diabetes in Adults chapter, p. S88).
A limited number of small, short-term studies conducted on the use of low-CHO diets (target <75 g/day) in people with type 1 diabetes have demonstrated modest adherence to the prescribed diets with improved A1C for those who can adhere. This style of diet can be an option for those motivated to be so restrictive (38,39). Of concern for those following a low-CHO diet is the effectiveness of glucagon in the treatment of hypoglycemia. In a small study, people with type 1 diabetes treated with continuous subcutaneous insulin infusion (CSII) therapy following a low-CHO diet for 1 week had a blunted response to a glucagon bolus (40,41). The long-term sustainability and safety of these diets remains uncertain.
Glycemic Index
The glycemic index (GI) provides an assessment of the quality of CHO-containing foods based on their ability to raise blood glucose (BG) (42). To decrease the glycemic response to dietary intake, low-GI CHO foods are exchanged for high-GI CHO foods. Detailed lists can be found in the International Tables of Glycemic Index and Glycemic Load Values (43).
Systematic reviews and meta-analyses of randomized trials and large individual randomized trials of interventions replacing high-GI foods with low-GI foods have shown clinically significant improvements in glycemic control over 2 weeks to 6 months in people with type 1 or type 2 diabetes (44–51). This dietary strategy has also been shown to improve postprandial glycemia and reduce high-sensitivity C-reactive protein (hsCRP) over 1 year in people with type 2 diabetes (48), reduce the number of hypoglycemic events over 24 to 52 weeks in adults and children with type 1 diabetes (47) and improve total cholesterol (TC) over 2 to 24 weeks in people with and without diabetes (46). Irrespective of the comparator, recent systematic reviews and meta-analyses have confirmed the beneficial effect of low-GI diets on glycemic control and blood lipids in people with diabetes (49–51). Other lines of evidence extend these benefits. A systematic review and meta-analysis of prospective cohort studies inclusive of people with diabetes showed that high GI and high glycemic load (GL) diets are associated with increased incidence of cardiovascular disease (CVD), when comparing the highest with the lowest exposures of GI and GL in women more than men over 6 to 25 years (52).
Dietary fibre
Dietary fibre includes the edible components of plant material that are resistant to digestion by human enzymes (nonstarch polysaccharides and lignin, as well as associated substances). They include fibres from commonly consumed foods as well as accepted novel fibres that have been synthesized or derived from agricultural by-products (53). DRIs specify an adequate intake (AI) for total fibre of 25 g/day and 38 g/day for women and men 19–50 years of age, respectively, and 21 g/day and 30 g/day for women and men ≥51 years of age, respectively (31). Although these recommendations do not differentiate between insoluble and soluble fibres or viscous and nonviscous fibres within soluble fibre, the evidence supporting metabolic benefit is greatest for viscous soluble fibre from different plant sources (e.g. beta-glucan from oats and barley, mucilage from psyllium, glucomannan from konjac mannan, pectin from dietary pulses, eggplant, okra and temperate climate fruits (apples, citrus fruits, berries, etc.). The addition of viscous soluble fibre has been shown to slow gastric emptying and delay the absorption of glucose in the small intestine, thereby improving postprandial glycemic control (54,55).
Systematic reviews, meta-analyses of randomized controlled trials and individual randomized controlled trials have shown that different sources of viscous soluble fibre result in improvements in glycemic control assessed as A1C or fasting blood glucose (FBG) (56–58) and blood lipids (59–61). A lipid-lowering advantage is supported by Health Canada-approved cholesterol-lowering health claims for the viscous soluble fibres from oats, barley and psyllium (62–64).
Despite contributing to stool bulking (65), insoluble fibre has failed to show similar metabolic advantages in randomized controlled trials in people with diabetes (56,66,67). These differences between soluble and insoluble fibre are reflected in the EURODIAB prospective complications study, which demonstrated a protective association of soluble fibre that was stronger than that for insoluble fibre in relation to nonfatal CVD, cardiovascular (CV) mortality and all-cause mortality in people with type 1 diabetes (68). However, this difference in the metabolic effects between soluble and insoluble fibre is not a consistent finding. A recent systematic review and meta-analysis of prospective cohort studies in people with and without diabetes did not show a difference in risk reduction between fibre types (insoluble, soluble) or fibre source (cereal, fruit, vegetable) (69). Given this inconsistency, mixed sources of fibre may be the ideal strategy. Interventions emphasizing high intakes of dietary fibre (≥20 g/1,000 kcal per day) from a combination of types and sources with a third or more provided by viscous soluble fibre (10 to 20 g/day) have shown important advantages for postprandial BG control and blood lipids, including the established therapeutic lipid target low-density lipoprotein cholesterol (LDL-C) (54,58,70) and, therefore, emphasizing fibre from mixed sources may help to ensure benefit.
Sugars
Added sugars, especially from fructose-containing sugars (high fructose corn syrup [HFCS], sucrose and fructose), have become a focus of intense public health concern. The main metabolic disturbance of fructose and sucrose in people with diabetes is an elevation of fasting triglycerides (TG) at doses >10% of total daily energy. A systematic review and meta-analysis of randomized controlled trials ≥2-weeks duration showed that added sugars from sucrose, fructose and honey in isocaloric substitution for starch have a modest fasting TG-raising effect in people with diabetes, which was not seen at doses ≤10% of total energy (71). Fructose-containing sugars either in isocaloric substitution for starch or under ad libitum conditions have not demonstrated an adverse effect on lipoproteins (LDL-C, TC, high-density lipoprotein cholesterol [HDL-C]), body weight or markers of glycemic control (A1C, FBG or fasting blood insulin) (71–73). Similar results have been seen for added fructose. Consumption of added fructose alone, in place of equal amounts of other sources of CHO (mainly starch), does not have adverse effects on body weight (74,75), BP (76), fasting TG (77,78), postprandial TG (79), markers of fatty liver (80) or uric acid (75,81). In fact, it may even lower A1C (75,82,83) in most people with diabetes. Although HFCS has not been formally tested in controlled trials involving people with diabetes, there is no reason to expect that it would give different results than sucrose. Randomized controlled trials of head-to-head comparisons of HFCS vs. sucrose at doses from the 5th to 95th percentile of United States population intake have shown no differences between HFCS and sucrose over a wide range of cardiometabolic outcomes in participants with overweight or obesity without diabetes (84–87).
Food sources of sugars may be a more important consideration than the type of sugar per se. A wide range of studies including people with and without diabetes have shown an adverse association of sugar-sweetened beverages (SSBs) with risk of hypertension and coronary heart disease when comparing the highest with the lowest levels of intake (88,89). These differences are most apparent when SSBs account for more than 10% of total energy and are likely mediated by the excess calories (88,89). This adverse relationship may be specific to SSBs as the same adverse relationship has not been shown for total sugars, sucrose, or fructose (90–97), fructose-containing sugars from fruit (79,98) or food sources of added sugars, such as whole grains and dairy products (yogurt) (98–101).
Fat
The DRIs do not specify an AI or RDA for total fat, monounsaturated fatty acids (MUFA), saturated fatty acids (SFA), or dietary cholesterol. AIs have only been set for the essential polyunsaturated fatty acids (PUFA): 12 g and 11 g per day for women and 17 g and 14 g per day for men aged 19-50 years and >51 years, respectively, for the n-6 PUFA linoleic acid and 1.1 g per day for women and 1.6 g per day for men aged >18 years for the n-3 PUFA alpha-linolenic acid (31). The quality of fat (type of fatty acids) has been shown to be a more important consideration than the quantity of fat for CV risk reduction. Dietary strategies have tended to focus on the reduction of saturated fatty acids (SFA) and dietary cholesterol. The prototypical diets are the United States National Cholesterol Education Program (NCEP) Step I (≤30% total energy as fat, ≤10% of energy as SFA) and Step II (≤7% of energy as SFA, dietary cholesterol ≤200 mg/day) diets (102). These diets have shown improvements in lipids and other CV risk factors compared with higher SFA and cholesterol control diets (103).
More recent analyses have assessed the relation of different fatty acids with CV outcomes. A systematic review and meta-analysis of prospective cohort studies inclusive of people with diabetes showed that diets low in trans fatty acids (TFA) are associated with less coronary heart disease (CHD) (104). Another systematic review and meta-analysis of randomized controlled clinical outcome trials involving people with and without diabetes showed that diets low in SFA decrease combined CV events (105). The benefit, however, was restricted to intakes of SFA <9% total energy and to the replacement of SFA with polyunsaturated fatty acids (PUFA) (105). Other analyses of the available clinical outcome trials suggest that the food sources of PUFA may even be more relevant with CV benefit restricted to mixed omega-3/omega-6 PUFA sources, such as soybean oil and canola oil (106). Pooled analyses of prospective cohort studies and large individual cohort studies also suggest that replacement of saturated fatty acids with high quality sources of monounsaturated fatty acids (MUFA) from olive oil, canola oil, avocado, nuts and seeds, and high quality sources of carbohydrates from whole grains and low GI index carbohydrate foods is associated with decreased incidence of CHD (107,108).
The food source of the saturated fatty acids being replaced, however, is another important consideration. Whereas adverse associations have been reliably established for meat as a food source of saturated fatty acids, the same has not been shown for some other food sources of saturated fatty acids (e.g. such as dairy products and plant fats from palm and coconut) (109).
A comprehensive review of long-chain omega-3 fatty acids (LC-PUFAs) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) from fish oils did not show an effect on glycemic control (110). Large randomized clinical outcome trials of supplementation with omega-3 LC-PUFAs do not support their use in people with diabetes (111–113). The Outcome Reduction with Initial Glargine lntervention (ORIGIN) trial failed to show a CV or mortality benefit of supplementation with omega-3 LC-PUFA in 12,536 people with prediabetes or type 2 diabetes (112). Subsequent systematic reviews and meta-analyses of randomized trials involving more than 75,000 participants with and without diabetes have failed to show a CV benefit of supplementation with long chain omega-3 PUFAs (114). The Study of Cardiovascular Events in Diabetes (ASCEND) in 15,480 people with diabetes free of CV disease (clinicaltrials.gov registration number NCT00135226) will provide more data on the outcomes of supplementation with omega-3 LC-PUFA in people with diabetes.
Although supplementation with omega-3 LC-PUFA has not been shown to be beneficial, consumption of fish may be. Prospective cohort analyses have shown higher consumption of fish, ranging from 1 to 3 servings per month to ≥2 servings/week of oily fish, was associated with reductions in coronary artery disease (CAD) (115), diabetic chronic kidney disease (CKD) in type 2 diabetes (116) and less albuminuria in type 1 diabetes (117).
Protein
The DRIs specify a recommended dietary allowance (RDA) for protein of 0.8 g per kg body weight for adult men and women >18 years of age (31). There is no evidence that the usual protein intake for most individuals (1 to 1.5 g per kg body weight per day), representing 15% to 20% of total energy intake, needs to be modified for people with diabetes (118). However, this intake in grams per kg per day should be maintained or increased with energy-reduced diets.
Protein quality has been shown to be another important consideration. A systematic review and meta-analysis of randomized controlled trials showed that replacement of animal protein with sources of plant protein improved A1C, FPG and fasting insulin in people with type 1 and type 2 diabetes over a median follow up of 8 weeks (119).
People with diabetes who have CKD should target a level of intake that does not exceed the RDA of 0.8 g per kilogram body weight per day (31), which has been shown to reduce end stage renal disease and mortality in people with type 1 diabetes and CKD (120) and improve albuminuria and A1C in people with CKD in diabetes (121). When using a low-protein diet, harm due to malnutrition should not be ignored (122). Both the quantity and quality (high biological value) of protein intake must be optimized to meet requirements for essential amino acids, necessitating adequate clinical and laboratory monitoring of nutritional status in the individual with diabetes and CKD. Greater incorporation of plant sources of protein may also require closer monitoring of potassium as CKD progresses.
Macronutrient substitutions
The ideal macronutrient distribution for the management of diabetes can be individualized. Based on evidence for chronic disease prevention and adequacy of essential nutrients, the DRIs recommend acceptable macronutrient distribution ranges (AMDRs) for macronutrients as a percentage of total energy. These include 45% to 65% energy for CHO, 10% to 35% energy for protein and 20% to 35% energy for fat, with 5% to 10% energy derived from linoleic acid and 0.6% to 1.2% energy derived from alpha linolenic acid (31).
There may be a benefit of substituting fat as MUFA for carbohydrate (123). A systematic review and meta-analysis of randomized controlled trials found that MUFA in isocaloric substitution for CHO (mean replacement of ~14% energy with a dietary macronutrient composition of 40% energy CHO, 33% energy fat, and 17% energy protein) did not reduce A1C but did improve FPG, body weight, systolic BP, TG and HDL-C in people with type 2 diabetes over an average follow up of 19 weeks (123). Similarly, the replacement of refined high-GI CHO with MUFA (14.5% total energy) or nuts (5% total energy) to affect a low glycemic load has been shown to improve A1C and lipids, including the established therapeutic lipid target LDL-C in people with type 2 diabetes over 3 months (124).
The effect of the replacement of fat with CHO depends on the quality of the CHO and the fat. Whereas the replacement of fat with refined high-GI CHO results in worsening of metabolic parameters in people with type 2 diabetes (125), the replacement of saturated fatty acids with low-GI CHO or whole grain sources is associated with decreased incident CHD in people with and without diabetes (107,108).
When protein is used to replace CHO, as in a high-protein diet, benefit has only been demonstrated when high-GI CHO are replaced. A 12-month randomized controlled trial in individuals with type 2 diabetes showed improved CV risk profile with a high-protein diet (30% energy protein, 40% energy CHO, 30% energy fat) vs. a high-CHO diet (15% energy protein, 55% energy CHO, 30% energy fat), in which the CHO were high GI. These differences were seen despite similar weight loss with normal renal function being maintained (126). In contrast, a 12-month randomized controlled trial comparing a high-protein diet (30% energy protein, 40% energy CHO, 30% energy fat) vs. a high-CHO low-GI diet (15% energy protein, 55% energy CHO, 30% energy fat) failed to show a difference between the diets (127). Rather, it was adherence to any 1 diet and the degree of energy restriction, not the variation in diet macronutrient composition, that was associated with the long-term improvement in glycemic control and cardiometabolic risk factors (127).
Adjustments in medication type and dosage may be required when embarking on a different macronutrient distribution (128) or energy reduction (129) to avoid hypoglycemia.
Intensive Lifestyle Intervention
Intensive lifestyle intervention (ILI) programs in diabetes usually consist of behavioural interventions combining dietary modification and increased physical activity. An interprofessional team, including registered dietitians, nurses and kinesiologists, usually leads the ILl programs, with the intensity of follow up varying from weekly to every 3 months with gradually decreasing contact as programs progress. Large, randomized clinical trials have shown benefit of ILl programs using different lifestyle approaches in diabetes. Twenty-year follow up of the China Da Qing Diabetes Prevention Outcome Study showed that 6 years of an ILl program targeting an increase in vegetable intake, decrease in alcohol and simple sugar intake, weight loss through energy restriction in participants with overweight or obesity, and an increase in leisure time physical activity (e.g. 30 minutes walking per day) reduced severe retinopathy by 47%, whereas nephropathy and neuropathy outcomes were not affected compared with usual care in high-risk people with impaired glucose tolerance (IGT) (130). After 23 years of follow up, the intervention group had a 41% reduction in CV mortality, 29% reduction in all cause-mortality and 45% reduction in progression to type 2 diabetes (131).
Analyses of the Look Action for Health in Diabetes (AHEAD) trial have shown that an ILl program targeting at least a 7% weight loss through a restriction in energy (1,200 to 1,800 total kcal/day based on initial weight), a reduction in fat (<30% of energy as total fat and <10% as saturated fat), an increase in protein (≥15% of energy) and an increase in physical activity (175 min/week with an intensity similar to brisk walking) produced sustained weight loss during 10 years follow up compared with diabetes support and education in persons with overweight and type 2 diabetes (132). However, it should be noted that analysis after 8 years showed that initial weight loss was attributable to reduction in both fat and lean mass, whereas weight regain was attributable only to fat mass, with continued decline in lean mass (133). Improvements in glycemic control and CV risk factors (BP, TG and HDL-C) were greatest at 1 year and diminished over time with the most sustainable reductions being in A1C, fitness and systolic BP (132). In 2012, the Look AHEAD trial was stopped early as it was determined that 11 years of an ILl did not decrease the occurrence of CV events compared to the control group and further intervention was unlikely to change this result. It was noted, however, that both groups had a lower number of CV events compared to previous studies of people with diabetes. Other studies of ILI have shown similar results (134,135).
Although the available trials suggest an overall short-term benefit of different ILl programs in people with diabetes, the feasibility of implementing an ILl program will depend on the availability of resources and access to an interprofessional team. Effects attenuate within 8 years and do not appear to provide lasting CV protection.
Dietary Patterns
A variety of dietary patterns have been studied for people with prediabetes and diabetes. An individual's values, preferences and treatment goals will influence the decision to use these dietary patterns.
Mediterranean dietary patterns
A Mediterranean diet primarily refers to a plant-based diet first described in the 1960s (136). General features include high consumption of fruits, vegetables, legumes, nuts, seeds, cereals and whole grains; moderate-to-high consumption of olive oil (as the principal source of fat); low-to-moderate consumption of dairy products, fish and poultry; low consumption of red meat; and low-to-moderate consumption of wine, mainly during meals (136,137). Systematic reviews and meta-analyses of randomized controlled feeding trials have shown that a Mediterranean-style dietary pattern improves glycemic control (50,138), and improves systolic BP, TC, HDL-C, TC:HDL-C ratio and TG in type 2 diabetes (139,140).
A low-CHO Mediterranean-style diet reduced A1C, delayed the need for antihyperglycemic drug therapy and increased rates of diabetes remission compared with a low-fat diet in overweight individuals with newly diagnosed type 2 diabetes at 8 years (141). Compared with a diet based on the American Diabetes Association recommendations, both traditional and low-CHO Mediterranean-style diets were shown to decrease A1C and TG, whereas only the low-CHO Mediterranean-style diet improved LDL-C and HDL-C at 1 year in persons with overweight and type 2 diabetes (142).
The Prevencion con Dieta Mediterranea (PREDIMED) study, a Spanish multicentre randomized trial of the effect of a Mediterranean diet supplemented with extra-virgin olive oil or mixed nuts compared with a low-fat American Heart Association (AHA) control diet, was stopped early due to significant benefit with reduction in major CV events in 7,447 participants at high CV risk (including 3,614 participants [49%] with type 2 diabetes) (143). Both types of Mediterranean diets were shown to reduce the incidence of major CV events by approximately 30% without any subgroup differences between participants with and without diabetes over a median follow up of 4.8 years (143) (see Cardiovascular Protection in People with Diabetes chapter, p. S162). Both the extra-virgin olive oil and mixed nuts arms of the PREDIMED trial also reduced risk of incident retinopathy. No effect on nephropathy was detected (144).
Vegetarian dietary patterns
Vegetarian dietary patterns include lacto-ovovegetarian, lactovegetarian, ovovegetarian and vegan dietary patterns. A low-fat, ad libitum vegan diet has been shown to be just as beneficial as conventional American Diabetes Association dietary guidelines in promoting weight loss and improving fasting BG and lipids over 74 weeks in adults with type 2 diabetes and, when taking medication changes into account, the vegan diet improved glycemia and plasma lipids more than the conventional diet (145). On both diets, weekly or biweekly nutrition and cooking instruction was provided by a dietitian or cooking instructor (145). Similarly, a calorie-restricted vegetarian diet was shown to improve BMI and LDL-C more than a conventional diet in people with type 2 diabetes (139). While both diets were effective in reducing A1C, more participants on the vegetarian diet had a decrease in antihyperglycemic medications compared to those on the conventional diet (43% vs. 5%, respectively). Subsequent systematic reviews and meta-analyses of the available randomized controlled trials have shown that vegetarian and vegan dietary patterns resulted in clinically meaningful improvements in A1C and FBG in people with type 1 and type 2 diabetes over 4 to 74 weeks (146,147), as well as body weight (148) and blood lipids (149) in people with and without diabetes over 3 to 74 weeks. Although most of these effects have been seen on high-CHO, low-fat vegetarian and vegan dietary patterns, there is evidence from the Eco-Atkins trial that these apply equally to low-CHO vegetarian dietary patterns (130 g/day [26% energy] CHO, 31% energy protein and 43% energy fat) for up to 6 months in individuals with overweight but without diabetes (150,151). A systematic review and meta-analysis of prospective cohort and cross-sectional observational studies showed a protective association between vegetarian dietary patterns and incident fatal and nonfatal CHD (152).
DASH and low-sodium dietary patterns
Dietary approaches to reducing BP have focused on sodium reduction and the Dietary Approaches to Stop Hypertension (DASH) dietary pattern. Although advice to the general population over 1 year of age is to achieve a sodium intake that meets the adequate intake (AI) target of 1,000 to 1,500 mg/day (depending on age, sex, pregnancy and lactation) (153), there is recent concern from prospective cohort studies that low-sodium intakes may be associated with increased mortality in people with type 1 (154) and type 2 diabetes (155).
The DASH dietary pattern does not target sodium reduction but rather emphasizes vegetables, fruits and low-fat dairy products, and includes whole grains, poultry, fish and nuts. It contains smaller amounts of red and processed meat, sweets, sugar-containing beverages, total and saturated fat, and cholesterol, and larger amounts of potassium, calcium, magnesium, dietary fibre and protein than typical Western diets (156,157). The DASH dietary pattern has been shown to lower systolic and diastolic BP compared with a typical American diet matched for sodium intake in people with and without hypertension, inclusive of people with well-controlled diabetes (156,157). These improvements in BP have been shown to hold at high (3,220 mg), medium (2,300 mg), and low (1,495 mg) levels of matched sodium intake (157). In addition to BP-lowering benefit, a systematic review and meta-analysis of randomized controlled trials showed that a DASH dietary pattern lowered lipids, including LDL-C in people with and without hypertension, some of whom had metabolic syndrome or diabetes (158).
In the only randomized controlled trial done exclusively in people with type 2 diabetes, a DASH dietary pattern compared with control diet for a moderate sodium intake (2,400 mg) was shown to decrease systolic and diastolic BP, A1C, FPG, weight, waist circumference, LDL-C and C-reactive protein (CRP) and to increase HDL-C over 8 weeks (159,160). A systematic review and meta-analysis of prospective cohort studies that included people with diabetes showed that adherence to a DASH dietary pattern was associated with a reduction in incident CVD (161).
Portfolio dietary pattern
The Portfolio Diet was conceived as a dietary portfolio of cholesterol-lowering foods, each with Federal Drug Administration (FDA) and/or Health Canada-approved health claims for cholesterol lowering or CV risk reduction. The 4 pillars of the Portfolio Diet include 2 g/day plant sterols (plant-sterol-containing margarines, supplements), 20 g/day viscous soluble fibres (gel-forming fibres from oats, barley, psyllium, konjac mannan, legumes, temperate climate fruits, eggplant, okra, etc.), 45 g/day plant protein (soy and pulses) and 45 g/day nuts (peanuts and tree nuts). Added to a low saturated fat NCEP Step II diet (≤7% saturated fat, ≤200 mg cholesterol), which reduces cholesterol by 5% to 10%, each component of the Portfolio Diet provides an additional 5% to 10% of LDL-C lowering. These small effects combine to provide a meaningful overall reduction in LDL-C lowering. The Portfolio Diet under conditions where all foods were provided has been shown to reduce LDL-C (~30%), hs-CRP (~30%) and calculated 10-year CVD risk by the Framingham Risk Score (~25%) in participants with hypercholesterolemia over 4 weeks (162). The reductions fell to 10% to 15% for LDL-C and 11% for 10-year CVD risk by the Framingham Risk Score (with greater effects in those who were more adherent) in a multicentre Canadian randomized controlled trial of effectiveness in which the Portfolio Diet was administered as dietary advice in participants with hypercholesterolemia over 6 months (163).
Although the Portfolio dietary pattern has not been formally tested in people with diabetes, each component has been shown individually to lower LDL-C in systematic reviews and meta-analyses of randomized controlled trials inclusive of people with diabetes (57,59–61,164–167). The results of the Combined Portfolio Diet and Exercise Study (PortfolioEx trial), a 3-year multicentre randomized controlled trial of the effect of the Portfolio Diet plus exercise on atherosclerosis, assessed by magnetic resonance imaging (MRI) in high CV risk people (ClinicalTrials.gov Identifier, NCT02481466), will provide important new data in people with diabetes, as approximately one-half of the participants will have type 2 diabetes.
Nordic dietary patterns
The Nordic Diet was developed as a Nordic translation of the Mediterranean, Portfolio, DASH and NCEP dietary patterns, using foods typically consumed as part of a traditional Nordic diet in the context of Nordic Nutrition Recommendations (168). It emphasizes ≥25% energy as whole-grain products, ≥175 g/day temperate fruits (apples and pears), ≥150 to 200 g/day berries (lingonberries and blueberry jam), ≥175 g/day vegetables, legumes (beans, peas, chickpeas and lentils), canola oil, ≥3 servings/week fatty fish (salmon, herring and mackerel), ≥2 servings/day low-fat dairy products, as well as several of the LDL-C-lowering foods common to the Portfolio Diet, including nuts (almonds), viscous fibres (oats, barley, psyllium), and vegetable protein (soy). The Nordic Diet has not been studied in people with diabetes; however, 3 high-quality randomized controlled trials have studied the effect of a Nordic Diet on glycemic control and other relevant cardiometabolic outcomes in people with central obesity or metabolic syndrome. These have shown improvements in body weight, insulin resistance, and lipids, including the therapeutically relevant LDL-C and non-HDL-C (169–171).
Popular weight-loss diets
Numerous popular weight-loss diets providing a range of macronutrient profiles are available to people with diabetes. Several of these diets, including the Atkins™, Zone™, Ornish™, Weight Watchers™ and Protein Power Lifeplan™ diets, have been subjected to investigation in longer-term, randomized controlled trials in participants with overweight or obesity that included some people with diabetes, although no available trials have been conducted exclusively in people with diabetes. A systematic review and meta-analysis of 4 trials of the Atkins™ diet and 1 trial of the Protein Power Lifeplan™ diet (a diet with a similar extreme CHO restriction) showed that these diets were no more effective than conventional energy-restricted, low-fat diets in inducing weight loss with improvements in TG and HDL-C offset by increases in TC and LDL-C for up to 1 year (172). The Protein Power Lifeplan™ diet, however, did show improved A1C compared with an energy-reduced, low-fat diet at 1 year in the subgroup with type 2 diabetes (173). The Dietary Intervention Randomized Controlled Trial (DIRECT) showed that the Atkins™ diet produced weight loss and improvements in the lipid profile compared with a calorie-restricted, low-fat conventional diet; however, its effects were not different from that of a calorie-restricted Mediterranean-style diet at 2 years (174). Furthermore, the Mediterranean-style diet had a more favourable effect on FPG at 2 years in the subgroup of participants with type 2 diabetes (174). Another trial comparing the Atkins™, Ornish™, Weight Watchers™ and Zone™ diets showed similar weight loss and improvements in the LDL-C:HDL-C ratio without effects on FPG at 1 year in participants with overweight or obesity, of whom 28% had diabetes (175). A network systematic review and meta-analysis comparing all available trials of popular diets that were ≥3 months found that weight loss differences between individual diets was minimal at 12 months in individuals with overweight or obesity with a range of metabolic phenotypes, including type 2 diabetes (36).
Diets Emphasizing Specific Foods
Dietary pulses and legumes
Dietary pulses, the dried seeds of nonoil seed legumes, include beans, peas, chickpeas, and lentils. This taxonomy does not include the oil-seed legumes (soy, peanuts) or fresh legumes (peas, beans). Systematic reviews and meta-analyses of randomized controlled trials found that diets high in dietary pulses, either alone or as part of low-GI or high-fibre diets, lowered fasting BG and/or glycated blood proteins, including A1C (176) and improved LDL-C, BP and body weight in people with and without diabetes (177–179). In people with type 2 diabetes, a small randomized crossover trial not captured in the census of these meta-analyses, found that substituting pulse-based foods for red meat (average increase of 5 servings/week of pulses vs. a decrease of 7 servings/week red meat) in the context of a NCEP diet resulted in reductions in FBG, fasting insulin, TG and LDL-C without significant change in body weight (180). A systematic review and meta-analysis of prospective cohort studies, inclusive of people with diabetes, showed that the intake of 4 weekly 100 g servings of legumes is associated with decreased incident total CHD (181).
Fruit and vegetables
Eating Well with Canada's Food Guide recommends up to 7 to 10 servings of fruit and vegetables per day (182). Individual randomized controlled trials have shown that supplementation with fresh or freeze dried fruits improves A1C over 6 to 8 weeks in individuals with type 2 diabetes (183,184). A novel and simple technique of encouraging intake of vegetables first and other CHOs last at each meal was successful in achieving better glycemic control (A1C) than an exchange-based meal plan after 24 months of follow up in people with type 2 diabetes (185). A systematic review and meta-analysis of randomized controlled trials also showed that fruit and vegetables (provided as either foods or supplements) improved diastolic BP over 6 weeks to 6 months in individuals with the metabolic syndrome, some of whom had prediabetes (186). In people with type 1 and type 2 diabetes, an intervention to increase the intake of fruit, vegetables and dairy that only succeeded in increasing the intake of fruits and vegetables, led to a similar improvement in diastolic blood pressure and to a clinically meaningful regression in carotid intima medial thickness over 1 year (187). Systematic reviews and meta-analyses of prospective cohort studies inclusive of people with diabetes have shown that higher intakes of fruit and vegetables (>5 servings/day), fruit alone (>3 servings/day) or vegetables alone (>4 servings/day) is associated with a decreased risk of CV and all-cause mortality (79). Although there is a need to understand better the advantages of different fruit and vegetables in people with diabetes, higher intake of total fruit and vegetables remains an important part of all healthy dietary patterns.
Nuts
Nuts include both peanuts (a legume) and tree nuts, such as almonds, walnuts, pistachios, pecans, Brazil nuts, cashews, hazelnuts, macadamia nuts and pine nuts. A systematic review and meta-analysis of 12 randomized controlled trials of at least 3 weeks duration found that diets enriched with nuts at a median dose of 56 g/day resulted in a small yet significant reduction in A1C and FPG in people with diabetes (188). Another systematic review and meta-analysis of 49 randomized controlled trials of the effect of nuts on metabolic syndrome criteria found that diets emphasizing nuts at a median dose of ~50 g/day decreased FPG and TG over a median follow up of 8 weeks in people with and without diabetes (189). An individual patient-level meta-analysis of 25 nut intervention trials of the effect of nuts on lipid outcomes in people with normolipidemia or hypercholesterolemia (including 1 trial in people with type 2 diabetes) also showed a dose-dependent reduction in blood lipids, including the established therapeutic target LDL-C (190).
The PREDIMED trial showed that the provision of mixed nuts (30 g/day) added to a Mediterranean diet compared with a low-fat control diet decreased major CV events by 30% over a median follow up of 4.8 years in high-CV risk participants, half of whom had type 2 diabetes (143). A systematic review and meta-analysis of prospective cohort studies in people with and without diabetes also showed that the intake of 4 weekly 28.4 g servings of nuts was associated with comparable reductions in fatal and nonfatal CHD (181).
Despite concerns that the high energy density of nuts may contribute to weight gain, systematic reviews of randomized controlled trials have failed to show an adverse effect of nuts on body weight and measures of adiposity when nuts are consumed as part of balanced, healthy dietary patterns (189,191).
Whole grains
Health Canada defines whole grains as those that contain all 3 parts of the grain kernel (bran, endosperm, germ) in the same relative proportions as they exist in the intact kernel. Health Canada recommends that at least half of all daily grain servings are consumed from whole grains (192). Sources of whole grains include both the cereal grains (e.g. wheat, rice, oats, barley, corn, wild rice, and rye) and pseudocereal grains (e.g. quinoa, amaranth and buckwheat) but not oil seeds (e.g. soy, flax, sesame seeds, poppy seeds). Systematic reviews and meta-analyses of randomized controlled trials have shown that whole grain interventions, specifically with whole grain sources containing the viscous soluble fibre beta-glucan, such as oats and barley, improve lipids, including TG and LDL-C, in people with and without diabetes over 2 to 16 weeks of follow up (193). Whole grains have also been shown to improve glycemic control. Whole grains from barley have shown improvements in fasting glucose in people with and without diabetes (57) and whole grains from oats have shown improvements in A1C and FPG in the subgroup with type 2 diabetes (194). In contrast, these advantages have not been seen for whole grain sources from whole wheat or wheat bran in people with type 2 diabetes (56,66,67). Systematic reviews and meta-analyses of prospective cohort studies have shown a protective association of total whole grains (where wheat is the dominant source) and total cereal fibre (as a proxy of whole grains) with incident CHD in people with and without diabetes (69,99). Although higher intake of all whole grains remains advisable (especially from oats and barley), more research is needed to understand the role of different sources of whole grains in people with diabetes.
Dairy products
Dairy products broadly include low- and full-fat milk, cheese, yogurt, other fermented products and ice cream. Evidence for the benefit of specific dairy products as singular interventions in the management of diabetes is inconclusive.
Systematic reviews and meta-analyses of randomized controlled trials of the effect of diets rich in either low- or full-fat dairy products have not shown any clear advantages for body weight, body fat, waist circumference, FPG or BP across individuals with different metabolic phenotypes (otherwise healthy, with overweight or obesity, or metabolic syndrome) (195,196). The comparator, however, may be an important consideration. Individual randomized controlled trials, which have assessed the effect of dairy products in isocaloric substitution with SSBs and foods, have shown advantages for visceral adipose tissue, systolic blood pressure and triglycerides in individuals with overweight or obesity over 6 months (197) and markers of insulin resistance in people with prediabetes over 6 weeks (198).
Other evidence from observational studies is suggestive of a weight loss and CV benefit. Large pooled analyses of the Harvard cohorts have shown that higher intakes of yogurt are associated with decreased body weight over 12 to 20 years of follow up in people with and without diabetes (98). Systematic reviews and meta-analyses of prospective cohort studies inclusive of people with diabetes have also shown a protective association of cheese with incident CHD; low-fat dairy products with incident CHD; and total, low-fat, and full-fat dairy products, and total milk with incident stroke over 5 to 26 years of follow up (199,200).
Special Considerations for People with Type 1 Diabetes and Type 2 Diabetes on Insulin
For persons on insulin, consistency in CHO intake (201) and spacing and regularity in meal consumption may help control BG levels (201–203). Inclusion of snacks as part of a person's meal plan should be individualized based on meal spacing, metabolic control, treatment regimen and risk of hypoglycemia, and should be balanced against the potential risk of weight gain (204,205).
The nutritional recommendations that reduce CV risk apply to both type 1 and type 2 diabetes. Studies have shown that people with type 1 diabetes tend to consume diets that are low in fibre, and high in protein and saturated fat (206). In addition, it was shown in the Diabetes Control and Complications Trial (DCCT), intensively treated individuals with type 1 diabetes showed worse diabetes control with diets high in total and saturated fat and low in CHO (207). Meals high in fat and protein may require additional insulin and, for those using CSII, the delivery of insulin may be best given over several hours (208). Algorithms for improved bolusing are under investigation. Heavy CHO loads (greater than 60 g) have been shown to result in greater glucose area under the curve and some risk of late postprandial hypoglycemia (209).
People with type 1 diabetes or type 2 diabetes requiring insulin, using a basal-bolus regimen, should adjust their insulin based on the CHO content of their meals, and inject their insulin within 15 minutes of eating with rapid-acting insulin analogues (208) and just prior to and if required up to 20 minutes after eating with faster-acting insulin aspart for optimal match between rapid insulin and glycemic meal rise (210) (see Glycemic Management of Type 1 Diabetes in Adults chapter, p. S80).
Intensive insulin therapy regimens that include multiple injections of rapid-acting insulin matched to CHO allow for flexibility in meal size and frequency (211,212). Improvements in A1C, BG and quality of life, as well as less requirement for insulin, can be achieved when individuals with type 1 diabetes (213) or type 2 diabetes (214) receive education on matching insulin to CHO content (e.g. CHO counting) (215,216). In doing so, dietary fibre and sugar alcohol should be subtracted from total CHO.
New interactive technologies, using mobile phones to provide information, CHO/insulin bolus calculations and telemedicine communications with care providers, have been shown to decrease both weight gain and the time required for education. They also improved individual quality of life and treatment satisfaction (217). Caution should be exercised in selection of smartphone bolus calculator apps for insulin calculation as there is a lack of regulation and surveillance, which may pose life-threatening risk and/or suboptimal control (218).
Other Considerations
Non-nutritive sweeteners
Sugar substitutes, which include high-intensity sweeteners and sugar alcohols, are regulated as food additives in Canada. Health Canada has approved the following high-intensity non-nutritive sweeteners for use in foods and chewing gum and/or as a tabletop sweetener: acesulfame potassium, aspartame, cyclamate, neotame, saccharin, steviol glycosides, sucralose, thaumatin and Monk fruit extract (219). Health Canada has set acceptable daily intake (ADI) values, which are expressed on a body weight basis and are considered safe daily intake levels over a lifetime (Table 2
Sugar alcohols approved for use in Canada include: erythritol, isomalt, lactitol, maltitol, mannitol, sorbitol, xylitol. There is no ADI for sugar alcohols (except for erythritol) as their use is considered self-limiting due to the potential for adverse gastrointestinal symptoms. They vary in the degree to which they are absorbed, and their conversion rate to glucose is slow, variable and usually minimal, and may have no significant effect on BG. Thus, matching rapid-acting insulin to the intake of sugar alcohols is not recommended (226). Although there are no long-term, randomized controlled trials of consumption of sugar alcohols by people with diabetes, consumption of up to 10 g/day by people with diabetes does not appear to result in adverse effects (227).
| Table 2 Acceptable daily intake of sweeteners |
|
|---|---|
| A1C, glycated hemoglobin; SMBG, self-monitoring of blood glucose. | |
| Sweetener | Acceptable daily intake (mg/kg body weight/day) |
| Acesulfame potassium | 15 |
| Aspartame | 40 |
| Cyclamate | 11 |
| Erythritol | 1,000 |
| Neotame | 2 |
| Saccharin | 5 |
| Sucralose | 8.8 |
| Tagatose | 80 |
| Thaumatin | 0.9 |
Meal replacements
Weight loss programs for people with diabetes may use partial meal replacement plans. Commercially available, portion-controlled, vitamin- and mineral-fortified meal replacement products usually replace 1 or 2 meals per day in these plans. Randomized controlled feeding trials have shown partial meal replacement plans result in comparable (228) or increased (229,230) weight loss compared with conventional reduced-calorie diets for up to 1 year with maintenance up to 86 weeks in people with type 2 diabetes and overweight. This weight loss results in greater improvements in glycemic control over 3 months to 34 weeks (230,231) and reductions in the need for antihyperglycemic medications up to 1 year without an increase in hypoglycemic or other adverse events (229–231). Meal replacements with differing macronutrient compositions designed for people with diabetes have shown no clear advantage, although studies are lacking (232,233).
Alcohol
The same precautions regarding alcohol consumption in the general population apply to people with diabetes (234). Alcohol consumption should be limited to ≤2 standard drinks per day and <10 drinks per week for women and ≤3 standard drinks per day or <15 drinks per week for men (1 standard drink: 10 g alcohol, 341 mL 5% alcohol beer, 43 ml 40% alcohol spirits, 142 ml 12% alcohol wine) (235). Chronic heavy consumption (>21 standard drinks/week for men and >14 standard drinks/week for women) is associated with increased risk of CVD, microvascular complications and all-cause mortality in people with type 2 diabetes (236), while light-to-moderate intake shows an inverse association with A1C (237). For people with type 1 diabetes, moderate consumption of alcohol with, or 2 or 3 hours after, an evening meal may result in delayed hypoglycemia the next morning after breakfast or as late as 24 hours after alcohol consumption (238,239) and may impede cognitive performance during mild hypoglycemia (240). The same concern may apply to sulphonylurea- and insulin-treated individuals with type 2 diabetes (241). Health-care professionals should discuss alcohol use with people with diabetes (242) to inform them of the potential weight gain and risks of hypoglycemia (241).
Vitamin and mineral supplements
People with diabetes should be encouraged to meet their nutritional needs by consuming a well-balanced diet by following Eating Well with Canada's Food Guide (182). Routine vitamin and mineral supplementation is generally not recommended. Supplementation with 10 μg (400 IU) vitamin D is recommended for people >50 years of age (182). Supplementation with folic acid (0.4 to 1.0 mg) is recommended for women who could become pregnant (182). The need for further vitamin and mineral supplements should be assessed on an individual basis. As vitamin and mineral supplements are regulated as natural health products (NHP) in Canada, the evidence for their therapeutic role in diabetes has been reviewed in the Complementary and Alternative Medicine for Diabetes chapter, p. S154.
Fasting and diabetes
Within the lay literature, intermittent energy restriction strategies for weight loss have become more prevalent. To date, there is limited evidence for these approaches with people with type 2 diabetes. In 1 preliminary study comparing continuous energy restriction (5,000–6,500 kJ/day) to 2 days of severe energy restriction (1,670–2,500 kJ/day) each week (the so called 5:2 approach) over a 12-week period, the 5:2 program, while as effective as continuous energy restriction for weight loss and glycemic control, required careful medication adjustment to protect against the risk of hypoglycemia on severe energy restriction days (243).
Ramadan
Traditionally, Muslims with type 1 and insulin-requiring type 2 diabetes have been exempted from participation in Ramadan fasting, due to concerns of hypo- and hyperglycemia. Similarly, people on non-insulin antihyperglycemic agents associated with hypoglycemia are also considered high risk for fasting. People with diabetes who wish to participate in Ramadan fasting are encouraged to consult with their diabetes health-care team 1 to 2 months prior to the start of Ramadan.
While evidence for the impact of Ramadan fasting in individuals with type 1 diabetes is limited, the literature suggests that in people with well-controlled type 1 diabetes, complications from fasting are rare. A reduction in the total daily dose of insulin can reduce the incidence of hypoglycemia. CSII therapy or the use of multiple daily injections with rapid-acting insulin taken with meals and basal insulin, combined with frequent self-monitoring of blood glucose (SMBG) can help reduce the risk of hypo- and hyperglycemia. Individuals with a history of severe hypoglycemia or hypoglycemia unawareness should be discouraged from participating in Ramadan fasting (210,244). More information on Diabetes and Ramadan management is available at http://www.daralliance.org/daralliance/wp-content/uploads/IDF-DAR-Practical-Guidelines_15-April-2016_low.pdf (210).
Food skills
While there is no universally agreed upon definition of food skills, it is generally thought that they are interdependent technical, mechanical, conceptual and perceptual skills that are necessary to safely select and plan, prepare, and store nutritious and culturally-acceptable meals and snacks (245–247). Several studies suggest that food preparation and cooking skills are declining globally (245,248,249). Over the past several decades, in Canada, there has been an increase in processed, pre-prepared and convenience foods being purchased and assembled rather than meals being prepared using whole, basic ingredients (250). To our knowledge, there are no studies that have investigated food skills in people with diabetes. Nevertheless, targeted interventions to improve the food skills of people living with diabetes are prudent given that food is central to managing glycemic control.
Recommendations
- People with diabetes should receive nutrition counselling by a registered dietitian to lower A1C levels [Grade B, Level 2 (3), for those with type 2 diabetes; Grade D, Consensus, for type 1 diabetes] and to reduce hospitalization rates [Grade C, Level 3 (8)].
- Nutrition education may be delivered in either a small group or one-on-one setting [Grade B, Level 2 (18)]. Group education should incorporate adult education principles, such as hands-on activities, problem solving, role playing and group discussions [Grade B, Level 2 (19)].
- Individuals with diabetes should be encouraged to follow Eating Well with Canada's Food Guide (182) in order to meet their nutritional needs [Grade D, Consensus].
- In people with overweight or obesity with diabetes, a nutritionally balanced, calorie-reduced diet should be followed to achieve and maintain a lower, healthier body weight [Grade A, Level 1A (29,30)].
- An intensive healthy behaviour intervention program, combining dietary modification and increased physical activity, may be used to achieve weight loss, improve glycemic control and reduce CV risk [Grade A, Level 1A (30)].
- In adults with diabetes, the macronutrient distribution as a percentage of total energy can range from 45% to 60% carbohydrate, 15% to 20% protein and 20% to 35% fat to allow for individualization of nutrition therapy based on preferences and treatment goals [Grade D, Consensus].
- People with type 2 diabetes should maintain regularity in timing and spacing of meals to optimize glycemic control [Grade D, Level 4 (203)].
- To reduce the risk of CVD, adults with diabetes should avoid trans fatty acids (TFA) [Grade D, Level 4 (104)] and consume less than 9% of total daily energy from saturated fatty acids (SFA) [Grade C, Level 2 (105)] replacing these fatty acids with polyunsaturated fatty acids (PUFA), particularly mixed n-3/n-6 sources [Grade C, Level 3 (105)], monounsaturated fatty acids (MUFA) from plant sources, whole grains [Grade D, Consensus (107)] or low-GI carbohydrates [Grade D, Consensus (108)].
- Adults with diabetes may substitute added sugars (sucrose, high fructose corn syrup, fructose, glucose) for other carbohydrates as part of mixed meals up to a maximum of 10% of total daily energy intake, provided adequate control of BG, lipids and body weight is maintained [Grade C, Level 3 (74,77,78,82)].
- Adults with type 1 and type 2 diabetes may aim to consume 30 to 50 g/day of dietary fibre with a third or more (10 to 20 g/day) coming from viscous soluble dietary fibre to improve glycemic control [Grade C, Level 3 (57)] and LDL-C [Grade C, Level 3 (54,57,59)], and reduce CV risk [Grade D, Level 4 (69)].
- Adults with diabetes should select carbohydrate food sources with a low-GI to help optimize glycemic control [Grade B, Level 2 (46,47) for type 1 diabetes; Grade B, Level 2 (32,44) for type 2 diabetes], to improve LDL-C [Grade C, Level 3 (49)] and to decrease CV risk [Grade D, Level 4 (52)].
- The following dietary patterns may be considered in people with type 2 diabetes, incorporating patient preferences, including:
- Mediterranean-style dietary pattern to reduce major CV events [Grade A, Level 1A (143)] and improve glycemic control [Grade B, Level 2 (50,139)].
- Vegan or vegetarian dietary pattern to improve glycemic control [Grade B, Level 2 (145,251)], body weight [Grade C, Level 3 (148)], and blood lipids, including LDL-C [Grade B, Level 2 (149)] and reduce myocardial infarction risk [Grade B, Level 2 (152)].
- DASH dietary pattern to improve glycemic control [Grade C, Level 2 (159)], BP [Grade D, Level 4 (156–159)], and LDL-C [Grade B, Level 2 (158,159)] and reduce major CV events [Grade B, Level 3 (161)].
- Dietary patterns emphasizing dietary pulses (e.g. beans, peas, chickpeas, lentils) to improve glycemic control [Grade B, Level 2 (176)], systolic BP [Grade C, Level 2 (178)] and body weight [Grade B, Level 2 (179)].
- Dietary patterns emphasizing fruit and vegetables to improve glycemic control [Grade B, Level 2 (183,184)] and reduce CV mortality [Grade C, Level 3 (79)].
- Dietary patterns emphasizing nuts to improve glycemic control [Grade B, Level 2 (188)], and LDL-C [Grade B, Level 2 (190)].
-
People with type 1 diabetes may be taught how to match insulin to carbohydrate quantity and quality [Grade C, Level 2 (213)] or they may maintain consistency in carbohydrate quantity and quality [Grade D, Consensus].
-
People with diabetes using insulin and/or insulin secretagogues should be educated about the risk of hypoglycemia resulting from alcohol [Grade C, Level 3 (239)], and should be advised on preventive actions, such as carbohydrate intake and/or insulin dose adjustments and increased BG monitoring [Grade D, Consensus].
Abbreviations:
A1C, glycated hemoglobin; AI, adequate intake; AMDRs, acceptable macronutrient distribution ranges; BG, blood glucose; BP, blood pressure; CAD, coronary artery disease; CHD, coronary heart disease; CHO, carbohydrate; CKD, chronic kidney disease; CRP, C-reactive protein; CSII, continuous subcutaneous insulin infusion; CV, cardiovascular, CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; DRIs, dietary reference intakes; FBG, fasting blood glucose; FPG, fasting plasma glucose; GI, glycemic index; HDL-C, high density lipoprotein cholesterol; HFCS, high fructose corn syrup; IFI, intensive lifestyle intervention; LC-PUFA, long-chain polyunsaturated fatty acid; LDL-C, low density lipoprotein cholesterol; MUFA, monounsaturated fatty acids; NCEP, National Cholesterol Education Program; NHP; natural health product; NPH, neutral protamine Hagedorn; PUFA, polyunsaturated fatty acids; RDA, recommended dietary allowance; SMBG, self-monitoring of blood glucose; SSBs, sugar-sweetened beverages; TC, total cholesterol; TFA, trans fatty acids; TG, triglycerides.
Other Relevant Guidelines
Literature Review Flow Diagram for Chapter 11: Nutrition Therapy
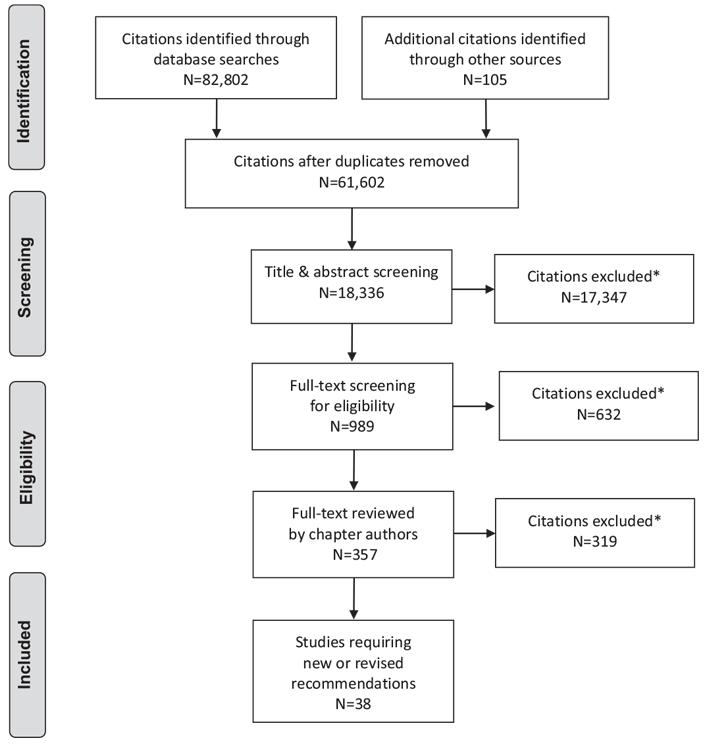
*Excluded based on: population, intervention/exposure, comparator/control or study design.
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi:10.1371/journal.pmed1000097 (252).
For more information, visit www.prisma-statement.org.
Author Disclosures
Dr. Sievenpiper reports grants from Canadian Institutes of Health Research (CIHR), Calorie Control Council, INC International Nut and Dried Fruit Council Foundation, The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), PSI Graham Farquharson Knowledge Translation Fellowship, Diabetes Canada Clinician Scientist Award, Banting & Best Diabetes Centre Sun Life Financial New Investigator Award, and CIHR INMD/CNS New Investigator Partnership Prize; grants and non-financial support from American Society for Nutrition (ASN), and Diabetes Canada; personal fees from mdBriefCase, Dairy Farmers of Canada, Canadian Society for Endocrinology and Metabolism (CSEM), GI Foundation, Pulse Canada, and Perkins Coie LLP; personal fees and non-financial support from Alberta Milk, PepsiCo, FoodMinds LLC, Memac Ogilvy & Mather LLC, Sprim Brasil, European Fruit Juice Association, The Ginger Network LLC, International Sweeteners Association, Nestlé Nutrition Institute, Mott's LLP, Canadian Nutrition Society (CNS), Winston & Strawn LLP, Tate & Lyle, White Wave Foods, and Rippe Lifestyle, outside the submitted work; membership in the International Carbohydrate Quality Consortium (ICQC) and on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes (EASD), Canadian Cardiovascular Society (CCS), and Canadian Obesity Network; appointments as an Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation; unpaid scientific advisor for the Food, Nutrition, and Safety Program (FNSP) and the Technical Committee on Carbohydrates of the International Life Science Institute (ILSI) North America; and spousal relationship with an employee of Unilever Canada. Dr. Chan reports grants from Danone Institute, Canadian Foundation for Dietetic Research, Alberta Livestock and Meat Agency, Dairy Farmers of Canada, Alberta Pulse Growers, and Western Canada Grain Growers, outside the submitted work; in addition, Dr. Chan has a patent No. 14/833,355 pending to the United States. Catherine Freeze reports personal fees from Dietitians of Canada and Government of Prince Edward Island, outside the submitted work. No other authors have anything to disclose.
References
- Pastors JG,WarshawH, Daly A, et al. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care 2002;25:608–13.
- Pi-Sunyer FX, Maggio CA, McCarron DA, et al. Multicenter randomized trial of a comprehensive prepared meal program in type 2 diabetes. Diabetes Care 1999;22:191–7.
- Franz MJ, Monk A, Barry B, et al. Effectiveness of medical nutrition therapy provided by dietitians in the management of non-insulin-dependent diabetes mellitus: A randomized, controlled clinical trial. J Am Diet Assoc 1995;95:1009–17.
- Kulkarni K, Castle G, Gregory R, et al. Nutrition practice guidelines for type 1 diabetes mellitus positively affect dietitian practices and patient outcomes. The Diabetes Care and Education Dietetic Practice Group. J Am Diet Assoc 1998;98:62–70, quiz 1-2.
- Gaetke LM, Stuart MA, Truszczynska H. A single nutrition counseling session with a registered dietitian improves short-term clinical outcomes for rural Kentucky patients with chronic diseases. J Am Diet Assoc 2006;106:109–12.
- Imai S, Kozai H, Matsuda M, et al. Intervention with delivery of diabetic meals improves glycemic control in patients with type 2 diabetes mellitus. J Clin Biochem Nutr 2008;42:59–63.
- Huang MC, Hsu CC, Wang HS, et al. Prospective randomized controlled trial to evaluate effectiveness of registered dietitian-led diabetes management on glycemic and diet control in a primary care setting in Taiwan. Diabetes Care 2010;33:233–9.
- Robbins JM, Thatcher GE, Webb DA, et al. Nutritionist visits, diabetes classes, and hospitalization rates and charges: The Urban Diabetes Study. Diabetes Care 2008;31:655–60.
- StatsCan. Immigration and ethnocultural diversity in Canada. Ottawa: Statistics Canada, 2011. Report No.: Catalogue no. 99-010-X2011001. http://www12.statcan.gc.ca/nhs-enm/2011/as-sa/99-010-x/99-010-x2011001-eng.pdf.
- Gougeon R, Sievenpiper JL, Jenkins D, et al. The transcultural diabetes nutrition algorithm: A Canadian perspective. Int J Endocrinol 2014;2014:151068.
- Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: A systematic review of randomized controlled trials. Diabetes Care 2001;24:561–87.
- Ash S, Reeves MM, Yeo S, et al. Effect of intensive dietetic interventions onweight and glycaemic control in overweight men with Type II diabetes: A randomised trial. Int J Obes Relat Metab Disord 2003;27:797–802.
- Vallis TM, Higgins-Bowser I, Edwards L. The role of diabetes education in maintaining lifestyle changes. Can J Diabetes 2005;29:193–202.
- Willaing I, Ladelund S, Jorgensen T, et al. Nutritional counselling in primary health care: A randomized comparison of an intervention by general practitioner or dietician. Eur J Cardiovasc Prev Rehabil 2004;11:513–20.
- Wilson C, Brown T, Acton K, et al. Effects of clinical nutrition education and educator discipline on glycemic control outcomes in the Indian health service. Diabetes Care 2003;26:2500–4.
- Brekke HK, Jansson PA, Lenner RA. Long-term (1- and 2-year) effects of lifestyle intervention in type 2 diabetes relatives. Diabetes Res Clin Pract 2005;70:225–34.
- Lemon CC, Lacey K, Lohse B, et al. Outcomes monitoring of health, behavior, and quality of life after nutrition intervention in adults with type 2 diabetes. J Am Diet Assoc 2004;104:1805–15.
- Rickheim PL,Weaver TW, Flader JL, et al. Assessment of group versus individual diabetes education: A randomized study. Diabetes Care 2002;25:269–74.
- TrentoM, Basile M, Borgo E, et al. A randomised controlled clinical trial of nurse-, dietitian- and pedagogist-led group care for the management of type 2 diabetes. J Endocrinol Invest 2008;31:1038–42.
- Pérez-Escamilla R, Hromi-Fiedler A, Vega-López S, et al. Impact of peer nutrition education on dietary behaviors and health outcomes among Latinos: A systematic literature review. J Nutr Educ Behav 2008;40:208–25.
- Ralston JD, Hirsch IB, Hoath J, et al. Web-based collaborative care for type 2 diabetes: A pilot randomized trial. Diabetes Care 2009;32:234–9.
- Marcy TR, Britton ML, Harrison D. Identification of barriers to appropriate dietary behavior in low-income patients with type 2 diabetes mellitus. Diabetes Ther 2011;2:9–19.
- Christensen NK, Terry RD, Wyatt S, et al. Quantitative assessment of dietary adherence in patients with insulin-dependent diabetes mellitus. Diabetes Care 1983;6:245–50.
- Toeller M, Klischan A, Heitkamp G, et al. Nutritional intake of 2868 IDDM patients from 30 centres in Europe. EURODIAB IDDM Complications Study Group. Diabetologia 1996;39:929–39.
- Glazier RH, Bajcar J, Kennie NR, et al. A systematic review of interventions to improve diabetes care in socially disadvantaged populations. Diabetes Care 2006;29:1675–88.
- Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2016;32:1263–82.
- Wing RR.Weight loss in the management of type 2 diabetes. In: Gerstein HC, Haynes B, eds. Evidence-based diabetes. Ontario: B.C, Decker Inc., 2000.
- Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50.
- KnowlerWC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403.
- The Look Ahead Research Group, Wing RR. Long term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes: Four year results of the Look AHEAD trial. Arch Intern Med 2010;170:1566–75.
- Food and Nutrition Board, Institute of Medicine of the National Academics. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids.Washington: The National Academies Press, 2005. https://www.nal.usda.gov/sites/default/files/fnic_uploads//energy_full_report.pdf.
- Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care 2006;29:1777–83.
- Kirk JK, Graves DE, Craven TE, et al. Restricted-carbohydrate diets in patients with type 2 diabetes: A meta-analysis. J Am Diet Assoc 2008;108:91–100.
- Dyson P. Low carbohydrate diets and type 2 diabetes: What is the latest evidence? Diabetes Ther 2015;6:411–24.
- van Wyk HJ, Davis RE, Davies JS. A critical review of low-carbohydrate diets in people with type 2 diabetes. Diabet Med 2016;33:148–57.
- Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: A meta-analysis. JAMA 2014;312:923–33.
- Yabe D, Iwasaki M, Kuwata H, et al. Sodium-glucose co-transporter-2 inhibitor use and dietary carbohydrate intake in Japanese individuals with type 2 diabetes: A randomized, open-label, 3-arm parallel comparative, exploratory study. Diabetes Obes Metab 2016;19:739–43.
- Krebs JD, Parry Strong A, Cresswell P, et al. A randomised trial of the feasibility of a low carbohydrate diet vs standard carbohydrate counting in adults with type 1 diabetes taking body weight into account. Asia Pac J Clin Nutr 2016;25:78–84.
- Nielsen JV, Gando C, Joensson E, et al. Low carbohydrate diet in type 1 diabetes, long-term improvement and adherence: A clinical audit. Diabetol Metab Syndr 2012;4:23.
- Ranjan A, Schmidt S, Damm-Frydenberg C, et al. Low-carbohydrate diet impairs the effect of glucagon in the treatment of insulin-induced mild hypoglycemia: A randomized crossover study. Diabetes Care 2017;40:132–5.
- Ranjan A, Schmidt S, Madsbad S, et al. Effects of subcutaneous, low-dose glucagon on insulin-induced mild hypoglycaemia in patients with insulin pump treated type 1 diabetes. Diabetes Obes Metab 2016;18:410–18.
- Jenkins DJ, Wolever TM, Taylor RH, et al. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am J Clin Nutr 1981;34:362–6.
- Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008;31:2281–3.
- Jenkins DJ, Kendall CW, McKeown-Eyssen G, et al. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: A randomized trial. JAMA 2008;300:2742–53.
- Brand-Miller J, Hayne S, Petocz P, et al. Low-glycemic index diets in the management of diabetes: A meta-analysis of randomized controlled trials. Diabetes Care 2003;26:2261–7.
- Opperman AM, Venter CS, Oosthuizen W, et al. Meta-analysis of the health effects of using the glycaemic index in meal-planning. Br J Nutr 2004;92:367–81.
- Thomas DE, Elliott EJ. The use of low-glycaemic index diets in diabetes control. Br J Nutr 2010;104:797–802.
- Wolever TM, Gibbs AL, Mehling C, et al. The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: No effect on glycated hemoglobin but reduction in C-reactive protein. Am J Clin Nutr 2008;87:114–25.
- Goff LM, Cowland DE, Hooper L, et al. Low glycaemic index diets and blood lipids: A systematic review and meta-analysis of randomised controlled trials. Nutr Metab Cardiovasc Dis 2013;23:1–10.
- Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr 2013;97:505–16.
- Wang Q, Xia W, Zhao Z, et al. Effects comparison between low glycemic index diets and high glycemic index diets on HbA1c and fructosamine for patients with diabetes: A systematic review and meta-analysis. Prim Care Diabetes 2015;9:362–9.
- Mirrahimi A, de Souza RJ, Chiavaroli L, et al. Associations of glycemic index and load with coronary heart disease events: A systematic review and metaanalysis of prospective cohorts. J Am Heart Assoc 2012;1:e000752.
- Policy for labelling and advertising of dietary fibre-containing food products. Ottawa: Bureau of Nutritional Sciences Food Directorate, Health Products and Food Branch: Health Canada, 2012. http://www.hc-sc.gc.ca/fn-an/alt_formats/pdf/legislation/pol/fibre-label-etiquetage-eng.pdf.
- Anderson JW, Randles KM, Kendall CW, et al. Carbohydrate and fiber recommendations for individuals with diabetes: A quantitative assessment and metaanalysis of the evidence. J Am Coll Nutr 2004;23:5–17.
- Grundy MM, Edwards CH, Mackie AR, et al. Re-evaluation of the mechanisms of dietary fibre and implications for macronutrient bioaccessibility, digestion and postprandial metabolism. Br J Nutr 2016;116:816–33.
- Vuksan V, Jenkins DJ, Spadafora P, et al. Konjac-mannan (glucomannan) improves glycemia and other associated risk factors for coronary heart disease in type 2 diabetes. A randomized controlled metabolic trial. Diabetes Care 1999;22:913–19.
- Tiwari U, Cummins E. Meta-analysis of the effect of beta-glucan intake on blood cholesterol and glucose levels. Nutrition 2011;27:1008–16.
- Post RE, Mainous AG 3rd, King DE, et al. Dietary fiber for the treatment of type 2 diabetes mellitus: A meta-analysis. J Am Board Fam Med 2012;25:16–23.
- Brown L, Rosner B, Willett WW, et al. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am J Clin Nutr 1999;69:30–42.
- Ho HV, Sievenpiper JL, Zurbau A, et al. A systematic review and metaanalysis of randomized controlled trials of the effect of barley beta-glucan on LDL-C, non-HDL-C and apoB for cardiovascular disease risk reductioni-iv. Eur J Clin Nutr 2016;70:1239–45.
- Ho HV, Sievenpiper JL, Zurbau A, et al. The effect of oat beta-glucan on LDLcholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: A systematic review and meta-analysis of randomised-controlled trials. Br J Nutr 2016;116:1369–82.
- Summary of Health Canada’s assessment of a health claim about food products containing psyllium an dblood cholesterol lowering. Ottawa: Bureau of Nutritional Sciences Food Directorate, Health Products and Food Branch: Health Canada, 2011. http://www.hc-sc.gc.ca/fn-an/alt_formats/pdf/label-etiquet/claims-reclam/assess-evalu/psyllium-cholesterol-eng.pdf.
- Summary of Health Canada’s assessment of a health claim about barley products and blood cholesterol lowering. Ottawa: Bureau of Nutritional Sciences Food Directorate, Health Products and Food Branch: Health Canada, 2012. http://www.hc-sc.gc.ca/fn-an/alt_formats/pdf/label-etiquet/claims-reclam/assessevalu/barley-orge-eng.pdf.
- Oat products and blood cholesterol lowering. Summary of assessment of a health claim about oat products and blood cholesterol lowering. Ottawa: Bureau of Nutritional Sciences Food Directorate, Health Products and Food Branch: Health Canada, 2010. http://www.hc-sc.gc.ca/fn-an/alt_formats/pdf/label-etiquet/claims-reclam/assess-evalu/oat_avoine-eng.pdf.
- Vuksan V, Jenkins AL, Jenkins DJ, et al. Using cereal to increase dietary fiber intake to the recommended level and the effect of fiber on bowel function in healthy persons consuming North American diets. AmJ Clin Nutr 2008;88:1256–62.
- Jenkins DJ, Kendall CW, Augustin LS, et al. Effect of wheat bran on glycemic control and risk factors for cardiovascular disease in type 2 diabetes. Diabetes Care 2002;25:1522–8.
- Jenkins DJ, Kendall CW, Augustin LS, et al. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: A randomized controlled trial. Arch Intern Med 2012;172:1653–60.
- Schoenaker DA, Toeller M, Chaturvedi N, et al. Dietary saturated fat and fibre and risk of cardiovascular disease and all-cause mortality among type 1 diabetic patients: The EURODIAB Prospective Complications Study. Diabetologia 2012;55:2132–41.
- Threapleton DE, Greenwood DC, Evans CEL, et al. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2013;347:f6879.
- Chandalia M, Garg A, Lutjohann D, et al. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl JMed 2000;342:1392–8.
- Te Morenga LA, Howatson AJ, Jones RM, et al. Dietary sugars and cardiometabolic risk: Systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am J Clin Nutr 2014;100:65–79.
- Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2013;346:e7492.
- Choo VL, Cozma AI, Viguiliouk E, et al. The effect of fructose-containing sugars on glycemic control: A systematic review and meta-analysis of controlled trials. FASEB J 2016;30:685.5.
- Sievenpiper JL, de Souza RJ, Mirrahimi A, et al. Effect of fructose on body weight in controlled feeding trials: A systematic review and meta-analysis. Ann Intern Med 2012;156:291–304.
- Sievenpiper JL, Chiavaroli L, de Souza RJ, et al. “Catalytic” doses of fructose may benefit glycaemic control without harming cardiometabolic risk factors: A small meta-analysis of randomised controlled feeding trials. Br J Nutr 2012;108:418–23.
- Ha V, Sievenpiper JL, De Souza RJ, et al. Effect of fructose on blood pressure: A systematic review and meta-analysis of controlled feeding trials. Hypertension 2012;59:787–95.
- Sievenpiper JL, Carleton AJ, Chatha S, et al. Heterogeneous effects of fructose on blood lipids in individuals with type 2 diabetes: Systematic review and meta-analysis of experimental trials in humans. Diabetes Care 2009;32:1930–7.
- Chiavaroli L, de Souza RJ, Ha V, et al. Effect of fructose on established lipid targets: A systematic review and meta-analysis of controlled feeding trials. J Am Heart Assoc 2015;4:e001700.
- Wang X, Ouyang Y, Liu J, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014;349:g4490.
- Chiu S, Sievenpiper JL, de Souza RJ, et al. Effect of fructose on markers of nonalcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr 2014;68:416–23.
- Wang DD, Sievenpiper JL, de Souza RJ, et al. The effects of fructose intake on serum uric acid vary among controlled dietary trials. J Nutr 2012;142:916–23.
- Cozma AI, Sievenpiper JL, de Souza RJ, et al. Effect of fructose on glycemic control in diabetes: A systematic review and meta-analysis of controlled feeding trials. Diabetes Care 2012;35:1611–20.
- Livesey G, Taylor R. Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: Meta-analyses and meta-regression models of intervention studies. Am J Clin Nutr 2008;88:1419–37.
- Lowndes J, Kawiecki D, Pardo S, et al. The effects of four hypocaloric diets containing different levels of sucrose or high fructose corn syrup on weight loss and related parameters. Nutr J 2012;11:55.
- Bravo S, Lowndes J, Sinnett S, et al. Consumption of sucrose and highfructose corn syrup does not increase liver fat or ectopic fat deposition in muscles. Appl Physiol Nutr Metab 2013;38:681–8.
- Lowndes J, Sinnett S, Pardo S, et al. The effect of normally consumed amounts of sucrose or high fructose corn syrup on lipid profiles, body composition and related parameters in overweight/obese subjects. Nutrients 2014;6:1128–44.
- Lowndes J, Sinnett S, Yu Z, et al. The effects of fructose-containing sugars on weight, body composition and cardiometabolic risk factors when consumed at up to the 90th percentile population consumption level for fructose. Nutrients 2014;6:3153–68.
- Xi B, Huang Y, Reilly KH, et al. Sugar-sweetened beverages and risk of hypertension and CVD: A dose-response meta-analysis. Br J Nutr 2015;113:709–17.
- Jayalath VH, de Souza RJ, Ha V, et al. Sugar-sweetened beverage consumption and incident hypertension: A systematic review and meta-analysis of prospective cohorts. Am J Clin Nutr 2015;102:914–21.
- Jayalath VH, Sievenpiper JL, de Souza RJ, et al. Total fructose intake and risk of hypertension: A systematic review and meta-analysis of prospective cohorts. J Am Coll Nutr 2014;33:328–39.
- Tasevska N, Park Y, Jiao L, et al. Sugars and risk of mortality in the NIH-AARP Diet and Health Study. Am J Clin Nutr 2014;99:1077–88.
- Liu S, Willett WC, Stampfer MJ, et al. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr 2000;71:1455–61.
- Beulens JW, de Bruijne LM, Stolk RP, et al. High dietary glycemic load and glycemic index increase risk of cardiovascular disease among middle-agedwomen: A population-based follow-up study. J Am Coll Cardiol 2007;50:14–21.
- Sieri S, Krogh V, Berrino F, et al. Dietary glycemic load and index and risk of coronary heart disease in a large italian cohort: The EPICOR study. Arch Intern Med 2010;170:640–7.
- Burger KN, Beulens JW, Boer JM, et al. Dietary glycemic load and glycemic index and risk of coronary heart disease and stroke in Dutch men and women: The EPIC-MORGEN study. PLoS ONE 2011;6:e25955.
- Burger KN, Beulens JW, van der Schouw YT, et al. Dietary fiber, carbohydrate quality and quantity, and mortality risk of individuals with diabetes mellitus. PLoS ONE 2012;7:e43127.
- Tsilas CS, de Souza RJ, Mejia SB, et al. Relation of total sugars, fructose and sucrose with incident type 2 diabetes: a systematic review and metaanalysis of prospective cohort studies. CMAJ 2017;189(20):E711–20. doi:10.1503/cmaj.160706.
- Mozaffarian D, Hao T, Rimm EB, et al. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404.
- Tang G,Wang D, Long J, et al. Meta-analysis of the association between whole grain intake and coronary heart disease risk. Am J Cardiol 2015;115:625–9.
- Qin LQ, Xu JY, Han SF, et al. Dairy consumption and risk of cardiovascular disease: An updated meta-analysis of prospective cohort studies. Asia Pac J Clin Nutr 2015;24:90–100.
- Smith JD, Hou T, Ludwig DS, et al. Changes in intake of protein foods, carbohydrate amount and quality, and long-term weight change: Results from 3 prospective cohorts. Am J Clin Nutr 2015;101:1216–24.
- Expert Panel on Detection Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 2001;285:2486–97.
- Yu-Poth S, Zhao G, Etherton T, et al. Effects of the National Cholesterol Education Program’s Step I and Step II dietary intervention programs on cardiovascular disease risk factors: A meta-analysis. Am J Clin Nutr 1999;69:632–46.
- de Souza RJ, Mente A, Maroleanu A, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015;351:h3978.
- Hooper L, Martin N, Abdelhamid A, et al. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev 2015;(6):CD011737.
- Ramsden CE, Zamora D, Leelarthaepin B, et al. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: Evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 2013;346:e8707.
- Li Y, Hruby A, Bernstein AM, et al. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: A prospective cohort study. J Am Coll Cardiol 2015;66:1538–48.
- Jakobsen MU, Dethlefsen C, Joensen AM, et al. Intake of carbohydrates compared with intake of saturated fatty acids and risk of myocardial infarction: Importance of the glycemic index. Am J Clin Nutr 2010;91:1764–8.
- de Oliveira Otto MC, Mozaffarian D, Kromhout D, et al. Dietary intake of saturated fat by food source and incident cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr 2012;96:397–404.
- McEwen B, Morel-Kopp MC, Tofler G, et al. Effect of omega-3 fish oil on cardiovascular risk in diabetes. Diabetes Educ 2010;36:565–84.
- Kromhout D, Giltay EJ, Geleijnse JM, et al. n–3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med 2010;363:2015–26.
- The ORIGIN Trial Investigators, Bosch J, Gerstein HC, et al. n–3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med 2012;367:309–18.
- Rizos EC, Ntzani EE, Bika E, et al. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA 2012;308:1024–33.
- Chowdhury R,Warnakula S, Kunutsor S, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann Intern Med 2014;160:398–406. . Erratum in: Ann Intern Med 2014;160:658.
- Hu FB, Cho E, Rexrode KM, et al. Fish and long-chain omega-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation 2003;107:1852–7.
- Sala-Vila A, Díaz-López A, Valls-Pedret C, et al. Dietary marine ω-3 fatty acids and incident sight-threatening retinopathy in middle-aged and older individuals with type 2 diabetes: Prospective investigation from the PREDIMED trial. JAMA Ophthalmol 2016;134:1142–9.
- Lee CC, Sharp SJ, Wexler DJ, et al. Dietary intake of eicosapentaenoic and docosahexaenoic acid and diabetic nephropathy: Cohort analysis of the diabetes control and complications trial. Diabetes Care 2010;33:1454–6.
- Hamdy O, Horton ES. Protein content in diabetes nutrition plan. Curr Diab Rep 2011;11:111–19.
- Viguiliouk E, Stewart SE, Jayalath VH, et al. Effect of replacing animal protein with plant protein on glycemic control in diabetes: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2015;7:9804–24.
- Hansen HP, Tauber-Lassen E, Jensen BR, et al. Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy. Kidney Int 2002;62:220–8.
- Pan Y, Guo LL, Jin HM. Low-protein diet for diabetic nephropathy: A metaanalysis of randomized controlled trials. Am J Clin Nutr 2008;88:660–6.
- Brodsky IG, Robbins DC, Hiser E, et al. Effects of low-protein diets on protein metabolism in insulin-dependent diabetes mellitus patients with early nephropathy. J Clin Endocrinol Metab 1992;75:351–7.
- Qian F, Korat AA, Malik V, et al. Metabolic effects of monounsaturated fatty acid-enriched diets compared with carbohydrate or polyunsaturated fatty acid-enriched diets in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Care 2016;39:1448–57.
- Jenkins DJ, Kendall CW, Vuksan V, et al. Effect of lowering the glycemic load with canola oil on glycemic control and cardiovascular risk factors: A randomized controlled trial. Diabetes Care 2014;37:1806–14.
- Kodama S, Saito K, Tanaka S, et al. Influence of fat and carbohydrate proportions on the metabolic profile in patients with type 2 diabetes: A metaanalysis. Diabetes Care 2009;32:959–65.
- Brinkworth GD, Noakes M, Parker B, et al. Long-term effects of advice to consume a high-protein, low-fat diet, rather than a conventionalweight-loss diet, in obese adults with type 2 diabetes: One-year follow-up of a randomised trial. Diabetologia 2004;47:1677–86.
- Larsen RN, Mann NJ, Maclean E, et al. The effect of high-protein, lowcarbohydrate diets in the treatment of type 2 diabetes: A 12 month randomised controlled trial. Diabetologia 2011;54:731–40.
- Haimoto H, Iwata M,Wakai K, et al. Long-term effects of a diet loosely restricting carbohydrates on HbA1c levels, BMI and tapering of sulfonylureas in type 2 diabetes: A 2-year follow-up study. Diabetes Res Clin Pract 2008;79:350–6.
- Davis NJ, Tomuta N, Schechter C, et al. Comparative study of the effects of a 1-year dietary intervention of a low-carbohydrate diet versus a low-fat diet onweight and glycemic control in type 2 diabetes. Diabetes Care 2009;32:1147–52.
- Gong Q, Gregg EW, Wang J, et al. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetesrelated microvascular complications: The China Da Qing Diabetes Prevention Outcome Study. Diabetologia 2011;54:300–7.
- Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: A 23-year follow-up study. Lancet Diabetes Endocrinol 2014;2:474–80.
- Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–54.
- Prior AM, Thapa M, Hua DH. Aldose reductase inhibitors and nanodelivery of diabetic therapeutics. Mini Rev Med Chem 2012;12:326–36.
- Mann JI, De Leeuw I, Hermansen K, et al. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr Metab Cardiovasc Dis 2004;14:373–94.
- Coppell KJ, Kataoka M, Williams SM, et al. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment—Lifestyle Over and Above Drugs in Diabetes (LOADD) study: Randomised controlled trial. BMJ 2010;341:c3337.
- WillettWC, Sacks F, Trichopoulou A, et al. Mediterranean diet pyramid: A cultural model for healthy eating. Am J Clin Nutr 1995;61:1402s–6s.
- Trichopoulou A, Costacou T, Bamia C, et al. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003;348:2599–608.
- Carter P, Achana F, Troughton J, et al. A Mediterranean diet improves HbA1c but not fasting blood glucose compared to alternative dietary strategies: A network meta-analysis. J Hum Nutr Diet 2014;27:280–97.
- Esposito K, Maiorino MI, Ceriello A, et al. Prevention and control of type 2 diabetes by Mediterranean diet: A systematic review. Diabetes Res Clin Pract 2010;89:97–102.
- Sleiman D, Al-Badri MR, Azar ST. Effect of Mediterranean diet in diabetes control and cardiovascular risk modification: A systematic review. Front Public Health 2015;3:69.
- Esposito K, Maiorino MI, Petrizzo M, et al. The effects of a Mediterranean diet on the need for diabetes drugs and remission of newly diagnosed type 2 diabetes: Follow-up of a randomized trial. Diabetes Care 2014;37:1824–30.
- Elhayany A, Lustman A, Abel R, et al. A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: A 1-year prospective randomized intervention study. Diabetes Obes Metab 2010;12:204–9.
- Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90.
- Díaz-López A, Babio N, Martínez-González MA, et al. Mediterranean diet, retinopathy, nephropathy, and microvascular diabetes complications: A post hoc analysis of a randomized trial. Diabetes Care 2015;38:2134–41.
- Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: A randomized, controlled, 74-wk clinical trial. Am J Clin Nutr 2009;89:1588s–96s.
- Yokoyama Y, Barnard ND, Levin SM, et al. Vegetarian diets and glycemic control in diabetes: A systematic review and meta-analysis. Cardiovasc Diagn Ther 2014;4:373–82.
- Viguiliouk E, Kahleová H, Rahelic´ D. editors. Vegetarian diets improve glycemic control in diabetes: A systematic review and meta-analysis of randomized controlled trials. Proceedings of the 34th international symposium on diabetes and nutrition. Prague: Czech Republic, 2016.
- Barnard ND, Levin SM, Yokoyama Y. A systematic review and meta-analysis of changes in body weight in clinical trials of vegetarian diets. J Acad Nutr Diet 2015;115:954–69.
- Wang F, Zheng J, Yang B, et al. Effects of vegetarian diets on blood lipids: A systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 2015;4:e002408.
- Jenkins DJ, Wong JM, Kendall CW, et al. The effect of a plant-based lowcarbohydrate (“Eco-Atkins”) diet on body weight and blood lipid concentrations in hyperlipidemic subjects. Arch Intern Med 2009;169:1046–54.
- Jenkins DJA, Wong JMW, Kendall CWC, et al. Effect of a 6-month vegan lowcarbohydrate (“Eco-Atkins”) diet on cardiovascular risk factors and body weight in hyperlipidaemic adults: A randomised controlled trial. BMJ Open 2014;4:003505.
- Dinu M, Abbate R, Gensini GF, et al. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr 2016 (in press).
- Food and Nutrition Board, Institute of Medicine. Dietary reference intakes for water, potassium, sodium, chloride, and sulfate.Washington: The National Academies Press, 2005. https://www.nal.usda.gov/sites/default/files/fnic_uploads//water_full_report.pdf.
- Thomas MC, Moran J, Forsblom C, et al. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care 2011;34:861–6.
- Ekinci EI, Clarke S, Thomas MC, et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care 2011;34:703–9.
- Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med 1997;336:1117–24.
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001;344:3–10.
- Siervo M, Lara J, Chowdhury S, et al. Effects of the dietary approach to stop hypertension (DASH) diet on cardiovascular risk factors: A systematic review and meta-analysis. Br J Nutr 2015;113:1–15.
- Azadbakht L, Fard NR, Karimi M, et al. Effects of the Dietary Approaches to Stop Hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients: A randomized crossover clinical trial. Diabetes Care 2011;34:55–7.
- Azadbakht L, Surkan PJ, Esmaillzadeh A, et al. The dietary approaches to stop hypertension eating plan affects C-reactiabnormalitiesve protein, coagulation, and hepatic function tests among type 2 diabetic patients. J Nutr 2011;141:1083–8.
- Schwingshackl L, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension score, and health outcomes: A systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 2015;115:780–800, e5.
- Jenkins DJ, Kendall CW, Marchie A, et al. Effects of a dietary portfolio of cholesterol-lowering foods vs lovastatin on serum lipids and C-reactive protein. JAMA 2003;290:502–10.
- Jenkins DJ, Jones PJ, Lamarche B, et al. Effect of a dietary portfolio of cholesterollowering foods given at 2 levels of intensity of dietary advice on serum lipids in hyperlipidemia: A randomized controlled trial. JAMA 2011;306:831–9.
- Lovejoy JC, Most MM, Lefevre M, et al. Effect of diets enriched in almonds on insulin action and serum lipids in adults with normal glucose tolerance or type 2 diabetes. Am J Clin Nutr 2002;76:1000–6.
- Jenkins DJ, Mirrahimi A, Srichaikul K, et al. Soy protein reduces serum cholesterol by both intrinsic and food displacement mechanisms. J Nutr 2010;140:2302s–11s.
- Tokede OA, Onabanjo TA, Yansane A, et al. Soya products and serum lipids: A meta-analysis of randomised controlled trials. Br J Nutr 2015;114:831–43.
- Ras RT, Geleijnse JM, Trautwein EA. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: Ameta-analysis of randomised controlled studies. Br J Nutr 2014;112:214–19.
- Nordic Council. Nordic nutrition recommendations 2004: integrating nutrition and physical acitivity. 4th edn. Arhus, Denmark: Nordic Council of Ministers, 2005.
- Adamsson V, Reumark A, Fredriksson IB, et al. Effects of a healthy Nordic diet on cardiovascular risk factors in hypercholesterolaemic subjects: A randomized controlled trial (NORDIET). J Intern Med 2011;269:150–9.
- Uusitupa M, Hermansen K, Savolainen MJ, et al. Effects of an isocaloric healthy Nordic diet on insulin sensitivity, lipid profile and inflammation markers in metabolic syndrome–arandomized study (SYSDIET). J InternMed 2013;274:52–66.
- Poulsen SK, Due A, Jordy AB, et al. Health effect of the NewNordic Diet in adults with increased waist circumference: A 6-mo randomized controlled trial. Am J Clin Nutr 2014;99:35–45.
- Nordmann AJ, Nordmann A, Briel M, et al. Effects of low-carbohydrate vs lowfat diets onweight loss and cardiovascular risk factors: Ameta-analysis of randomized controlled trials. Arch Intern Med 2006;166:285–93.
- Stern L, Iqbal N, Seshadri P, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: One-year follow-up of a randomized trial. Ann Intern Med 2004;140:778–85.
- Shai I, Schwarzfuchs D, Henkin Y, et al.Weight Loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 2008;359:229–41.
- Dansinger ML, Gleason JA, Griffith JL, et al. Comparison of the Atkins, Ornish, WeightWatchers, and Zone diets for weight loss and heart disease risk reduction: A randomized trial. JAMA 2005;293:43–53.
- Sievenpiper JL, Kendall CW, Esfahani A, et al. Effect of non-oil-seed pulses on glycaemic control: A systematic reviewand meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia2009;52:1479–95.
- Ha V, Sievenpiper JL, de Souza RJ, et al. Effect of dietary pulse intake on established therapeutic lipid targets for cardiovascular risk reduction: A systematic reviewandmeta-analysis of randomized controlled trials. CMAJ 2014;186:E252–62.
- Jayalath VH, de Souza RJ, Sievenpiper JL, et al. Effect of dietary pulses on blood pressure: A systematic review and meta-analysis of controlled feeding trials. Am J Hypertens 2014;27:56–64.
- Kim SJ, de Souza RJ, Choo VL, et al. Effects of dietary pulse consumption on body weight: A systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 2016;103:1213–23.
- Hosseinpour-Niazi S, Mirmiran P, Hedayati M, et al. Substitution of red meat with legumes in the therapeutic lifestyle change diet based on dietary advice improves cardiometabolic risk factors in overweight type 2 diabetes patients: A cross-over randomized clinical trial. Eur J Clin Nutr 2015;69:592–7.
- Afshin A, Micha R, Khatibzadeh S, et al. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: A systematic review and meta-analysis. Am J Clin Nutr 2014;100:278–88.
- Health Canada. Eating well with Canada’s food guide. Ottawa: Health Products and Food Branch, Office of Nutrition and Promotion, 2007. Report No.: Publication H39e166/1990E. http://www.hc-sc.gc.ca/fn-an/food-guide-aliment/index-eng.php.
- Moazen S, Amani R, Homayouni Rad A, et al. Effects of freeze-dried strawberry supplementation on metabolic biomarkers of atherosclerosis in subjects with type 2 diabetes: A randomized double-blind controlled trial. Ann Nutr Metab 2013;63:256–64.
- Hegde SV, Adhikari P, Nandini M, et al. Effect of daily supplementation of fruits on oxidative stress indices and glycaemic status in type 2 diabetes mellitus. Complement Ther Clin Pract 2013;19:97–100.
- Imai S, Matsuda M, Hasegawa G, et al. A simple meal plan of “eating vegetables before carbohydrate”was more effective for achieving glycemic control than an exchange-based meal plan in Japanese patients with type 2 diabetes. Asia Pac J Clin Nutr 2011;20:161–8.
- Shin JY, Kim JY, Kang HT, et al. Effect of fruits and vegetables on metabolic syndrome: A systematic reviewand meta-analysis of randomized controlled trials. Int J Food Sci Nutr 2015;66:416–25.
- Petersen KS, Clifton PM, Blanch N, et al. Effect of improving dietary quality on carotid intima media thickness in subjects with type 1 and type 2 diabetes: A 12-mo randomized controlled trial. Am J Clin Nutr 2015;102:771–9.
- Viguiliouk E, Kendall CW, Blanco Mejia S, et al. Effect of tree nuts on glycemic control in diabetes: A systematic reviewand meta-analysis of randomized controlled dietary trials. Endocrinol Metab Clin North Am 2014;9:e103376.
- Blanco Mejia S, Kendall CW, Viguiliouk E, et al. Effect of tree nuts onmetabolic syndrome criteria: A systematic reviewand meta-analysis of randomised controlled trials. BMJ Open 2014;4:e004660.
- Sabate J, Oda K, Ros E. Nut consumption and blood lipid levels: A pooled analysis of 25 intervention trials. Arch Intern Med 2010;170:821–7.
- Flores-Mateo G, Rojas-Rueda D, Basora J, et al. Nut intake and adiposity:Metaanalysis of clinical trials. Am J Clin Nutr 2013;97:1346–55.
- Whole grains—get the facts. Ottawa: Health Canada, 2013. http://www.hc-sc.gc.ca/fn-an/nutrition/whole-grain-entiers-eng.php.
- Hollænder PL, Ross AB, Kristensen M. Whole-grain and blood lipid changes in apparently healthy adults: A systematic reviewand meta-analysis of randomized controlled studies. Am J Clin Nutr 2015;102:556–72.
- Bao L, Cai X, Xu M, et al. Effect of oat intake on glycaemic control and insulin sensitivity: A meta-analysis of randomised controlled trials. Br J Nutr 2014;112:457–66.
- Chen M, Pan A, Malik VS, et al. Effects of dairy intake on body weight and fat: Ameta-analysis of randomized controlled trials. Am J Clin Nutr 2012;96:735–47.
- Benatar JR, Sidhu K, Stewart RA. Effects of high and lowfat dairy food on cardiometabolic risk factors: A meta-analysis of randomized studies. PLoS ONE 2013;8:e76480.
- Maersk M, Belza A, Stødkilde-Jørgensen H, et al. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: A 6-mo randomized intervention study. Am J Clin Nutr 2012;95:283–9.
- Maki KC, Nieman KM, Schild AL, et al. Sugar-wweetened product consumption alters glucose homeostasis compared with dairy product consumption in men and women at risk of type 2 diabetes mellitus. J Nutr 2015;145:459–66.
- Alexander DD, Bylsma LC, Vargas AJ, et al. Dairy consumption and CVD: A systematic reviewand meta-analysis—CORRIGENDUM. Br J Nutr 2016;115:2268.
- de Goede J, Soedamah-Muthu SS, Pan A, et al. Dairy consumption and risk of stroke: A systematic reviewand updated dose-response meta-analysis of prospective cohort studies. J Am Heart Assoc 2016;5:pii: e002787.
- Wolever TM, Hamad S, Chiasson JL, et al. Day-to-day consistency in amount and source of carbohydrate intake associated with improved blood glucose control in type 1 diabetes. J Am Coll Nutr 1999;18:242–7.
- Clement S. Diabetes self-management education. Diabetes Care 1995;18:1204–14.
- Savoca MR, Miller CK, Ludwig DA. Food habits are related to glycemic control among people with type 2 diabetes mellitus. J AmDiet Assoc 2004;104:560–6.
- Kalergis M, Schiffrin A, Gougeon R, et al. Impact of bedtime snack composition on prevention of nocturnal hypoglycemia in adults with type 1 diabetes undergoing intensive insulin management using lispro insulin before meals: A randomized, placebo-controlled, crossover trial. Diabetes Care 2003;26:9–15.
- Arnold L, Mann JI, Ball MJ. Metabolic effects of alterations in meal frequency in type 2 diabetes. Diabetes Care 1997;20:1651–4.
- Leroux C, Brazeau AS, Gingras V, et al. Lifestyle and cardiometabolic risk in adults with type 1 diabetes: A review. Can J Diabetes 2014;38:62–9.
- Delahanty LM, Nathan DM, Lachin JM, et al. Association of diet with glycated hemoglobin during intensive treatment of type 1 diabetes in the Diabetes Control and Complications Trial. Am J Clin Nutr 2009;89:518–24.
- Bell KJ, Smart CE, Steil GM, et al. Impact of fat, protein, and glycemic index on postprandial glucose control in type 1 diabetes: Implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care 2015;38:1008–15.
- Marran KJ, Davey B, Lang A, et al. Exponential increase in postprandial bloodglucose exposure with increasing carbohydrate loads using a linear carbohydrateto- insulin ratio. S Afr Med J 2013;103:461–3.
- International Diabetes Federation, DAR International Alliance. Diabetes and Ramadan: Practical guidelines. Brussels: (IDF) IDF, 2016. http://www.daralliance.org/daralliance/wp-content/uploads/IDF-DAR-Practical -Guidelines_15-April-2016_low.pdf.
- Tunbridge FK, Home PD, MurphyM, et al. Does flexibility at mealtimes disturb blood glucose control on a multiple insulin injection regimen? Diabet Med 1991;8:833–8.
- DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: Dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002;325:746.
- Scavone G, Manto A, Pitocco D, et al. Effect of carbohydrate counting andmedical nutritional therapy on glycaemic control in type 1 diabetic subjects: A pilot study. Diabet Med 2010;27:477–9.
- Bergenstal RM, Johnson M, Powers MA, et al. Adjust to target in type 2 diabetes: Comparison of a simple algorithmwith carbohydrate counting for adjustment of mealtime insulin glulisine. Diabetes Care 2008;31:1305–10.
- Gillespie SJ, Kulkarni KD, Daly AE. Using carbohydrate counting in diabetes clinical practice. J Am Diet Assoc 1998;98:897–905.
- Kelley DE. Sugars and starch in the nutritional management of diabetes mellitus. Am J Clin Nutr 2003;78:858s–64s.
- Rossi MC, Nicolucci A, Di Bartolo P, et al. Diabetes Interactive Diary: A new telemedicine system enabling flexible diet and insulin therapy while improving quality of life: An open-label, international, multicenter, randomized study. Diabetes Care 2010;33:109–15.
- Huckvale K, Adomaviciute S, Prieto JT, et al. Smartphone apps for calculating insulin dose: A systematic assessment. BMC Med 2015;13(1).
- List of permitted sweeteners (list of permitted food additives). Ottawa: Health Canada, 2016. http://www.hc-sc.gc.ca/fn-an/securit/addit/list/archive-9- sweetener-edulcorant-2017-04-27-eng.php.
- Gougeon R, Spidel M, Lee K, et al. Canadian Diabetes AssociationNationalNutrition Committee technical review: Nonnutritive intense sweeteners in diabetes management. Can J Diabetes 2004;28:385*99.
- Maki KC, Curry LL, Reeves MS, et al. Chronic consumption of rebaudioside A, a steviol glycoside, in men and women with type 2 diabetes mellitus. Food Chem Toxicol 2008;46(Suppl. 7):S47–53.
- Barriocanal LA, Palacios M, Benitez G, et al. Apparent lack of pharmacological effect of steviol glycosides used as sweeteners in humans.Apilot study of repeated exposures in some normotensive and hypotensive individuals and in Type 1 and Type 2 diabetics. Regul Toxicol Pharmacol 2008;51:37–41.
- Azad NA,Mielniczuk L. A call for collaboration: Improving cardiogeriatric care. Can J Cardiol 2016;32:1041–4.
- Narain A, Kwok CS, Mamas MA. Soft drinks and sweetened beverages and the risk of cardiovascular disease and mortality: A systematic review and metaanalysis. Int J Clin Pract 2016;70:791–805.
- Rogers PJ, Hogenkamp PS, de Graaf C, et al. Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int J Obes (Lond) 2016;40:381–94.
- Wang YM, van Eys J. Nutritional significance of fructose and sugar alcohols. Annu Rev Nutr 1981;1:437–75.
- Wolever TMS, Pickarz A, Hollands M, et al. Sugar alcohols and diabetes: A review. Can J Diabetes 2002;26:356–62.
- Heymsfield SB, van Mierlo CA, van der Knaap HC, et al. Weight management using a meal replacement strategy:Meta and pooling analysis from six studies. Int J Obes Relat Metab Disord 2003;27:537–49.
- Li Z, Hong K, Saltsman P, et al. Long-term efficacy of soy-based meal replacements vs an individualized diet plan in obese type II DM patients: Relative effects on weight loss, metabolic parameters, and C-reactive protein. Eur J Clin Nutr 2005;59:411–18.
- Cheskin LJ, Mitchell AM, Jhaveri AD, et al. Efficacy of meal replacements versus a standard food-based diet forweight loss in type 2 diabetes: A controlled clinical trial. Diabetes Educ 2008;34:118–27.
- Yip I, Go VL, DeShields S, et al. Liquid meal replacements and glycemic control in obese type 2 diabetes patients. Obes Res 2001;9(Suppl. 4):341s–7s.
- McCargar LJ, Innis SM, Bowron E, et al. Effect of enteral nutritional products differing in carbohydrate and fat on indices of carbohydrate and lipid metabolism in patients with NIDDM. Mol Cell Biochem 1998;188:81–9.
- Lansink M, van Laere KM, Vendrig L, et al. Lower postprandial glucose responses at baseline and after 4 weeks use of a diabetes-specific formula in diabetes type 2 patients. Diabetes Res Clin Pract 2011;93:421–9.
- Stockwell T, Zhao J, Thomas G. Should alcohol policies aim to reduce total alcohol consumption? Newanalyses of Canadian drinking patterns. Addict Res Theory 2009;17:135–51.
- Butt P, Beimess D, Stockwell T, et al. Alcohol and health in Canada: A summary of evidence and guidelines for low-risk drinking. Ottawa: Canadian Centre on Substance Abuse, 2011. http://www.ccsa.ca/Resource%20Library/2011-Summaryof- Evidence-and-Guidelines-for-Low-Risk%20Drinking-en.pdf.
- Blomster JI, Zoungas S, Chalmers J, et al. The relationship between alcohol consumption and vascular complications and mortality in individuals with type 2 diabetes. Diabetes Care 2014;37:1353–9.
- Ahmed AT, Karter AJ,Warton EM, et al. The relationship between alcohol consumption and glycemic control among patients with diabetes: The Kaiser Permanente Northern California Diabetes Registry. J Gen Intern Med 2008;23:275–82.
- Kerr D, Macdonald IA, Heller SR, et al. Alcohol causes hypoglycaemic unawareness in healthy volunteers and patients with type 1 (insulin-dependent) diabetes. Diabetologia 1990;33:216–21.
- Richardson T, Weiss M, Thomas P, et al. Day after the night before: Influence of evening alcohol on risk of hypoglycemia in patients with type 1 diabetes. Diabetes Care 2005;28:1801–2.
- Cheyne EH, Sherwin RS, Lunt MJ, et al. Influence of alcohol on cognitive performance during mild hypoglycaemia; implications for type 1 diabetes. Diabet Med 2004;21:230–7.
- Pietraszek A, Gregersen S, Hermansen K. Alcohol and type 2 diabetes. A review. Nutr Metab Cardiovasc Dis 2010;20:366–75.
- Gallagher A, Connolly V, Kelly WF. Alcohol consumption in patients with diabetes mellitus. Diabet Med 2001;18:72–3.
- Carter S, Clifton PM, Keogh JB. The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res Clin Pract 2016;122:106–12.
- Alabbood MH, Ho KW, Simons MR. The effect of Ramadan fasting on glycaemic control in insulin dependent diabetic patients: A literature review. Diabetes Metab Syndr 2016;11:83–7.
- Chenhall C, Healthy Living Issue Group (HLIG) of the Pan-Canadian Public Health Network. Improving cooking and food preparation skills. A synthesis paper of the evidence to inform program and policy development. Ottawa: Canada Go, 2010. Report No.: Cat.: H164-123/1-2010E-PDF. https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/fn-an/alt_formats/pdf/nutrition/childenfant/cfps-acc-synthes-eng.pdf.
- Desjardins E. Making something out of nothing: Food literacy among youth, young pregnant women and young parents who are at risk for poor health. Toronto: Public Health Ontario, 2013. https://www.publichealthontario.ca/en/ServicesAndTools/Documents/LDCP/LDCP.Food.Skills_Report_WEB_FINAL.pdf.
- Food skills: Definitions, influences and relationship with health. Cork, Ireland: SafeFood (Food Safety Promotion Board), 2014.
- Slater J. Is cooking dead? The state of home economics food and nutrition education in a Canadian province. Int J Consum Stud 2013;37:617–24.
- Nelson SA, Corbin MA, Nickols-Richardson SM. A call for culinary skills education in childhood obesity-prevention interventions: Current status and peer influences. J Acad Nutr Diet 2013;113:1031–6.
- Moubarac JC, Batal M, Martins AP, et al. Processed and ultra-processed food products: Consumption trends in Canada from 1938 to 2011. Can J Diet Pract Res 2014;75:15–21.
- Kahleova H, Matoulek M, Malinska H, et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes. Diabet Med 2011;28:549–59.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews andmeta-analyses: The PRISMA statement. PLoSMed 2009;6:e1000097.
*The Canadian Diabetes Association is the registered owner of the name Diabetes Canada. All content on guidelines.diabetes.ca, CPG Apps and in our online store remains exactly the same. For questions, contact communications@diabetes.ca.



